J Korean Soc Transplant.
2018 Jun;32(2):26-30. 10.4285/jkstn.2018.32.2.26.
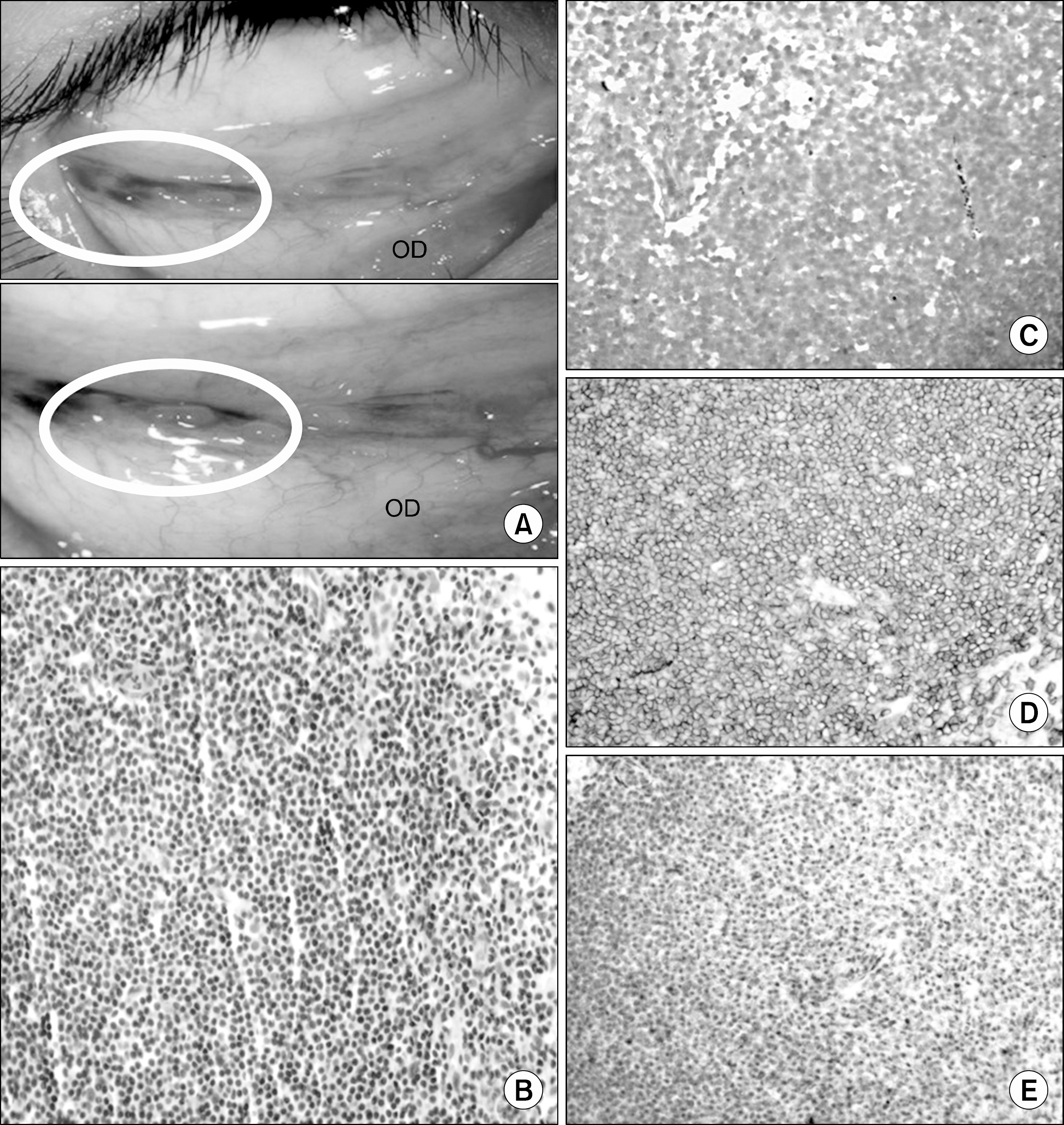
Bilateral Conjunctival Mucosa-Associated Lymphoid Tissue Type Lymphoma in a Kidney Transplant Recipient
- Affiliations
-
- 1Division of Nephrology, Department of Internal Medicine, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. chungbh@catholic.ac.kr
- 2Division of Hematology, Department of Internal Medicine, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 2414445
- DOI: http://doi.org/10.4285/jkstn.2018.32.2.26
Abstract
- Lymphoproliferative disorder in a posttransplant setting has emerged as a difficult problem in kidney transplantation (KT). Lymphoma involving adnexa of the eye has rarely been reported due to scarcity of lymphoreticular tissue in the ocular area. This report presents a case of a 37-year-old KT recipient who was diagnosed with conjunctival mucosa-associated lymphoid tissue lymphoma with a chief complaint of seeing black spots. Unlike other post-transplant lymphoproliferative diseases associated with the Epstein-Barr virus (EBV) reactivation via immunosuppression, the lesion was not related to the virus. The patient received radiotherapy with concomitant conversion from the tacrolimus to the sirolimus. Overall, the results presented herein indicate lymphoma may be an important differential diagnosis when KT recipients complain of ocular discomfort.
MeSH Terms
Figure
Reference
-
1). Bosly A., Coiffier B. Recent data on the epidemiology of non-Hodgkin lymphoma. Groupe d'Etudes des Lymphomes de l'Adulte (GELA). Pathol Biol (Paris). 1997. 45:449–52.2). Hsi ED., Singleton TP., Swinnen L., Dunphy CH., Alkan S. Mucosa-associated lymphoid tissue-type lymphomas occurring in post-transplantation patients. Am J Surg Pathol. 2000. 24:100–6.
Article3). Le Meur Y., Pontoizeau-Potelune N., Jaccard A., Paraf F., Leroux-Robert C. Regression of a gastric lymphoma of mucosa-associated lymphoid tissue after eradication of Helicobacter pylori in a kidney graft recipient. Am J Med. 1999. 107:530.4). Shehab TM., Hsi ED., Poterucha JJ., Gunaratnam NT., Fontana RJ. Helicobacter pylori-associated gastric MALT lymphoma in liver transplant recipients. Transplantation. 2001. 71:1172–5.
Article5). Vardiman JW. The World Health Organization (WHO) classification of tumors of the hematopoietic and lymphoid tissues: an overview with emphasis on the myeloid neoplasms. Chem Biol Interact. 2010. 184:16–20.
Article6). Smith JM., Rudser K., Gillen D., Kestenbaum B., Seliger S., Weiss N, et al. Risk of lymphoma after renal transplantation varies with time: an analysis of the United States Renal Data System. Transplantation. 2006. 81:175–80.
Article7). Achuthan R., Bell SM., Leek JP., Roberts P., Horgan K., Markham AF, et al. Novel translocation of the BCL10 gene in a case of mucosa associated lymphoid tissue lymphoma. Genes Chromosomes Cancer. 2000. 29:347–9.8). Enno A., O'Rourke J., Braye S., Howlett R., Lee A. Antigen-dependent progression of mucosa-associated lymphoid tissue (MALT)-type lymphoma in the stomach. Effects of antimicrobial therapy on gastric MALT lymphoma in mice. Am J Pathol. 1998. 152:1625–32.9). Hamoudi RA., Appert A., Ye H., Ruskone-Fourmestraux A., Streubel B., Chott A, et al. Differential expression of NF-kappaB target genes in MALT lymphoma with and without chromosome translocation: insights into molecular mechanism. Leukemia. 2010. 24:1487–97.10). Marshall BJ., Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984. 1:1311–5.
Article11). Gibson SE., Swerdlow SH., Craig FE., Surti U., Cook JR., Nalesnik MA, et al. EBV-positive extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue in the posttransplant setting: a distinct type of posttransplant lymphoproliferative disorder? Am J Surg Pathol. 2011. 35:807–15.12). Suarez F., Lortholary O., Hermine O., Lecuit M. Infection-associated lymphomas derived from marginal zone B cells: a model of antigen-driven lymphoproliferation. Blood. 2006. 107:3034–44.
Article13). Bates WD., Gray DW., Dada MA., Chetty R., Gatter KC., Davies DR, et al. Lymphoproliferative disorders in Oxford renal transplant recipients. J Clin Pathol. 2003. 56:439–46.
Article14). Goldfarb JM., Larson ML., Venugopal P., Gregory SA. Posttransplant lymphoproliferative disorder: extranodal marginal zone lymphoma occurring after renal transplantation. Clin Adv Hematol Oncol. 2006. 4:600–4.15). Douglas RS., Goldstein SM., Katowitz JA., Gausas RE., Ibarra MS., Tsai D, et al. Orbital presentation of posttransplantation lymphoproliferative disorder: a small case series. Ophthalmology. 2002. 109:2351–5.16). Taylor AL., Marcus R., Bradley JA. Post-transplant lymphoproliferative disorders (PTLD) after solid organ transplantation. Crit Rev Oncol Hematol. 2005. 56:155–67.
Article17). Morscio J., Dierickx D., Tousseyn T. Molecular pathogenesis of B-cell posttransplant lymphoproliferative disorder: what do we know so far? Clin Dev Immunol. 2013. 2013:150835.
Article18). Craig FE., Johnson LR., Harvey SA., Nalesnik MA., Luo JH., Bhattacharya SD, et al. Gene expression profiling of Epstein-Barr virus-positive and -negative monomorphic B-cell posttransplant lymphoproliferative disorders. Diagn Mol Pathol. 2007. 16:158–68.
Article19). Al-Mansour Z., Nelson BP., Evens AM. Post-transplant lymphoproliferative disease (PTLD): risk factors, diagnosis, and current treatment strategies. Curr Hematol Malig Rep. 2013. 8:173–83.
Article20). Opelz G., Dohler B. Lymphomas after solid organ transplantation: a collaborative transplant study report. Am J Transplant. 2004. 4:222–30.
Article21). Bhatia S., Paulino AC., Buatti JM., Mayr NA., Wen BC. Curative radiotherapy for primary orbital lymphoma. Int J Radiat Oncol Biol Phys. 2002. 54:818–23.
Article22). Bolek TW., Moyses HM., Marcus RB Jr., Gorden L 3rd., Maiese RL., Almasri NM, et al. Radiotherapy in the management of orbital lymphoma. Int J Radiat Oncol Biol Phys. 1999. 44:31–6.
Article23). Goda JS., Gospodarowicz M., Pintilie M., Wells W., Hodgson DC., Sun A, et al. Long-term outcome in localized extranodal mucosa-associated lymphoid tissue lymphomas treated with radiotherapy. Cancer. 2010. 116:3815–24.
Article24). Stafford SL., Kozelsky TF., Garrity JA., Kurtin PJ., Leavitt JA., Martenson JA, et al. Orbital lymphoma: radiotherapy outcome and complications. Radiother Oncol. 2001. 59:139–44.
Article25). Tsang RW., Gospodarowicz MK., Pintilie M., Wells W., Hodgson DC., Sun A, et al. Localized mucosa-associated lymphoid tissue lymphoma treated with radiation therapy has excellent clinical outcome. J Clin Oncol. 2003. 21:4157–64.
Article26). Lee JL., Kim MK., Lee KH., Hyun MS., Chung HS., Kim DS, et al. Extranodal marginal zone B-cell lymphomas of mucosa-associated lymphoid tissue-type of the orbit and ocular adnexa. Ann Hematol. 2005. 84:13–8.
Article27). Ashrafi F., Shahidi S., Ebrahimi Z., Mortazavi M. Outcome of rapamycin therapy for post-transplant-lymphoproliferative disorder after kidney transplantation: case series. Int J Hematol Oncol Stem Cell Res. 2015. 9:26–32.28). Cullis B., D'Souza R., McCullagh P., Harries S., Nicholls A., Lee R, et al. Sirolimus-induced remission of posttrans-plantation lymphoproliferative disorder. Am J Kidney Dis. 2006. 47:e67–72.
Article29). Boratynska M., Smolska D. Inhibition of mTOR by sirolimus induces remission of post-transplant lymphoproliferative disorders. Transpl Int. 2008. 21:605–8.30). Nelson BP., Nalesnik MA., Bahler DW., Locker J., Fung JJ., Swerdlow SH. Epstein-Barr virus-negative post-transplant lymphoproliferative disorders: a distinct entity? Am J Surg Pathol. 2000. 24:375–85.31). McKelvie PA., McNab A., Francis IC., Fox R., O'Day J. Ocular adnexal lymphoproliferative disease: a series of 73 cases. Clin Exp Ophthalmol. 2001. 29:387–93.
Article32). Oh YK., Ha CS., Samuels BI., Cabanillas F., Hess MA., Cox JD. Stages I-III follicular lymphoma: role of CT of the abdomen and pelvis in follow-up studies. Radiology. 1999. 210:483–6.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Bilateral Conjunctival Mucosa-Associated Lymphoid Tissue (MALT) Lymphoma Misdiagnosed as Allergic Conjunctivitis
- A Case of Bilateral Conjunctival Malignant Lymphoma Misdiagnosed as Allergic Conjunctivitis
- Malignant Lymphoma of Mucosa-associated Lymphoid Tissue Arising in the Conjunctiva
- Long-Term Outcomes after Cryotherapy for Conjunctival Mucosa-Associated Lymphoid Tissue Lymphomas
- Primary Conjunctival Malignant Lymphoma of Mucosa-Associated Lymophoid Tissue