Korean J Physiol Pharmacol.
2018 Jul;22(4):409-417. 10.4196/kjpp.2018.22.4.409.
Supplementing punicalagin reduces oxidative stress markers and restores angiogenic balance in a rat model of pregnancy-induced hypertension
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Sichuan Academy of Medical Sciences, Sichuan Provincial People's Hospital, Chengdu City, Sichuan 610072, China. MEveliadraf@yahoo.com
- 2Department of Obstetrics and Gynecology, Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan 646099, China.
- KMID: 2414265
- DOI: http://doi.org/10.4196/kjpp.2018.22.4.409
Abstract
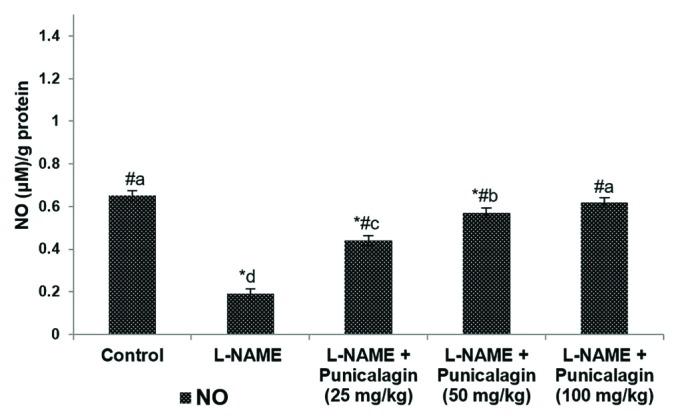
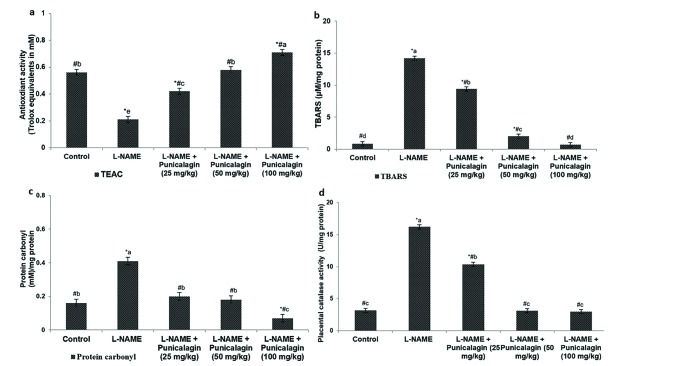
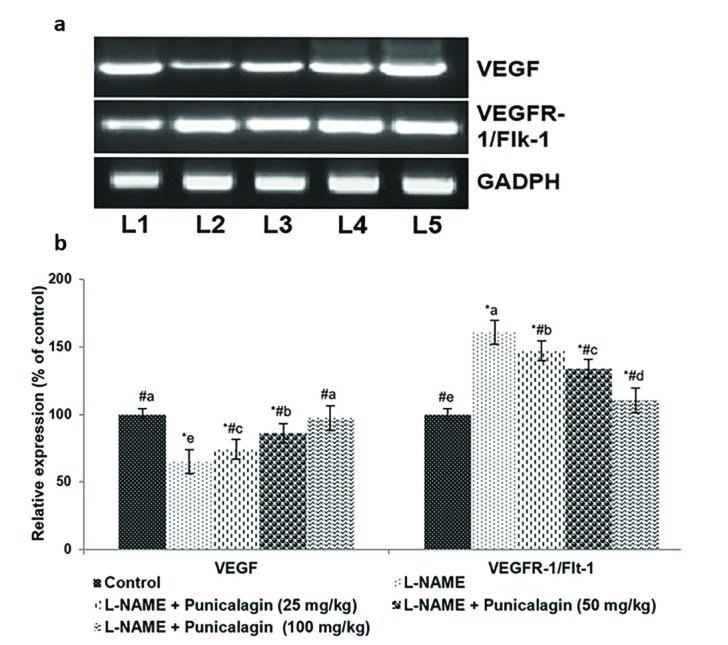
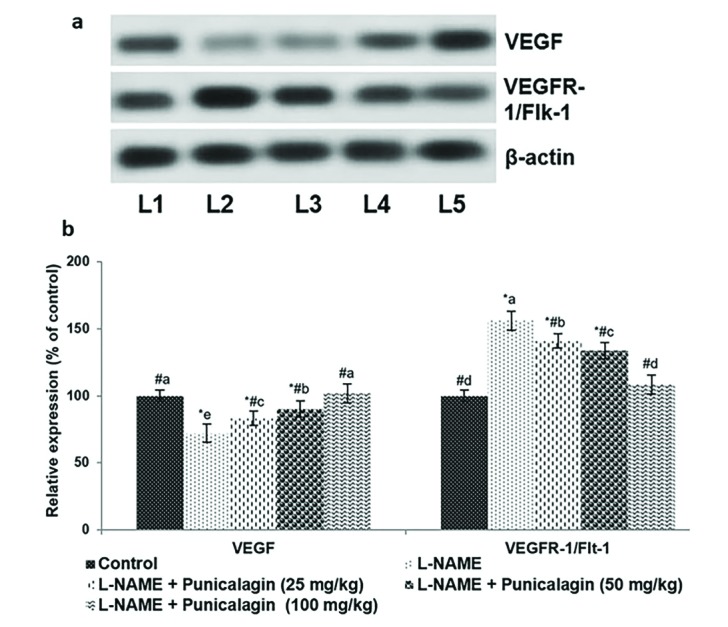
- Pre-eclampsia (PE) is a pregnancy disorder that is characterised by severe hypertension and increased risks of foetal and maternal mortality. The aetiology of PE not completely understood; however, maternal nutrition and oxidative stress play important roles in the development of hypertension. The treatment options for PE are currently limited to anti-hypertensive drugs. Punicalagin, a polyphenol present in pomegranate juice, has a range of bioactive properties. The effects of supplementation with punicalagin on angiogenesis and oxidative stress in pregnant rats with induced hypertension were investigated. The pregnant rats were randomly divided into five experimental groups (n=12 per group). Hypertension was induced using an oral dose of NG-nitro-L-arginine methyl ester (L-NAME, 50 mg/kg/day) on days 14-19 of pregnancy. Punicalagin (25, 50 or 100 mg/kg) was given orally on days 14-21 of pregnancy. Punicalagin treatment at the tested doses significantly reduced diastolic, systolic, and mean arterial blood pressure in L-NAME treated rats from day 14. Punicalagin also restored angiogenic balance by increasing the expression of vascular endothelial growth factor and downregulating vascular endothelial growth factor receptor-1/fms-like tyrosine kinase-1. Punicalagin, significantly increased the placental nitric oxide levels as compared to PE group. The increased levels of oxidative stress in rats with PE were markedly decreased by treatment with punicalagin. Punicalagin at the tested doses markedly (p < 0.05) enhanced the placental antioxidant capacity in L-NAME-treated rats. The raised catalase activity observed following L-NAME induction was significantly (p < 0.05) and restored to normal activity levels in punicalagin treatment. Further, 100 mg dose of punicalagin exhibited higher protective effects as compared to lower doses of 25 and 50 mg. This study shows that supplementation with punicalagin decreased blood pressure and oxidative stress and restored angiogenic balance in pregnant rats with induced PE.
MeSH Terms
-
Animals
Antihypertensive Agents
Arterial Pressure
Blood Pressure
Catalase
Female
Hypertension
Hypertension, Pregnancy-Induced*
Maternal Mortality
Models, Animal*
NG-Nitroarginine Methyl Ester
Nitric Oxide
Oxidative Stress*
Pre-Eclampsia
Pregnancy
Punicaceae
Rats*
Tyrosine
Vascular Endothelial Growth Factor A
Antihypertensive Agents
Catalase
NG-Nitroarginine Methyl Ester
Nitric Oxide
Tyrosine
Vascular Endothelial Growth Factor A
Figure
Reference
-
1. Roberts JM, Escudero C. The placenta in preeclampsia. Pregnancy Hypertens. 2012; 2:72–83. PMID: 22745921.
Article2. Henao DE, Saleem MA. Proteinuria in preeclampsia from a podocyte injury perspective. Curr Hypertens Rep. 2013; 15:600–605. PMID: 24214687.
Article3. August P, Lindheimer M. Pathophysiology of preeclampsia. In : Laragh J, Brenner BM, editors. Hypertension. New York: Raven Press;1995. p. 2407–2426.4. Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005; 365:785–799. PMID: 15733721.
Article5. Bijvank SW, Visser W, Duvekot JJ, Steegers EA, Edens MA, Roofthooft DW, Vulto AG, Hanff LM. Ketanserin versus dihydralazine for the treatment of severe hypertension in early-onset preeclampsia: a double blind randomized controlled trial. Eur J Obstet Gynecol Reprod Biol. 2015; 189:106–111. PMID: 25892082.
Article6. Zhang J, Meikle S, Trumble A. Severe maternal morbidity associated with hypertensive disorders in pregnancy in the United States. Hypertens Pregnancy. 2003; 22:203–212. PMID: 12909005.
Article7. Backes CH, Markham K, Moorehead P, Cordero L, Nankervis CA, Giannone PJ. Maternal preeclampsia and neonatal outcomes. J Pregnancy. 2011; 2011:214365. PMID: 21547086.
Article8. Hamilton BE, Martin JA, Osterman MJ, Curtin SC, Matthews TJ. Births: final data for 2014. Natl Vital Stat Rep. 2015; 64:1–64.9. Jido TA, Yakasai IA. Preeclampsia: a review of the evidence. Ann Afr Med. 2013; 12:75–85. PMID: 23713013.
Article10. Burton GJ, Jauniaux E. Oxidative stress. Best Pract Res Clin Obstet Gynaecol. 2011; 25:287–299. PMID: 21130690.
Article11. Bhale DV, Hivre MD, Mahat RK, Bujurge AA. Comparative study of serum malondialdehyde levels as marker of oxidative stress in patients of pregnancy-induced hypertension and controls. J Med Sci. 2014; 1:53–55.12. Sutherland MR, Bertagnolli M, Lukaszewski MA, Huyard F, Yzydorczyk C, Luu TM, Nuyt AM. Preterm birth and hypertension risk: the oxidative stress paradigm. Hypertension. 2014; 63:12–18. PMID: 24144644.13. de Lucca L, Gallarreta FMP, de Lima Gonçalves T. Oxidative stress markers in pregnant women with preeclampsia. Am J Med Biol Res. 2015; 3:68–73.14. Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003; 111:649–658. PMID: 12618519.
Article15. Gilbert JS, Verzwyvelt J, Colson D, Arany M, Karumanchi SA, Granger JP. Recombinant vascular endothelial growth factor 121 infusion lowers blood pressure and improves renal function in rats with placentalischemia-induced hypertension. Hypertension. 2010; 55:380–385. PMID: 20026764.
Article16. Ohkuchi A, Hirashima C, Matsubara S, Takahashi K, Matsuda Y, Suzuki M. Threshold of soluble fms-like tyrosine kinase 1/placental growth factor ratio for the imminent onset of preeclampsia. Hypertension. 2011; 58:859–866. PMID: 21947468.
Article17. Makris A, Thornton C, Thompson J, Thomson S, Martin R, Ogle R, Waugh R, McKenzie P, Kirwan P, Hennessy A. Uteroplacental ischemia results in proteinuric hypertension and elevated sFLT-1. Kidney Int. 2007; 71:977–984. PMID: 17377512.
Article18. Gilbert JS, Babcock SA, Granger JP. Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increased soluble fms-like tyrosine kinase-1 expression. Hypertension. 2007; 50:1142–1147. PMID: 17923588.
Article19. Wang Y, Walsh SW. Antioxidant activities and mRNA expression of superoxide dismutase, catalase, and glutathione peroxidase in normal and preeclamptic placentas. J Soc Gynecol Investig. 1996; 3:179–184.
Article20. Sedeek MH, Wang Y, Granger JP. Increased oxidative stress in a rat model of preeclampsia. Am J Hypertens. 2004; 17:142A.
Article21. Bridges JP, Gilbert JS, Colson D, Gilbert SA, Dukes MP, Ryan MJ, Granger JP. Oxidative stress contributes to soluble fms-like tyrosine kinase-1 induced vascular dysfunction in pregnant rats. Am J Hypertens. 2009; 22:564–568. PMID: 19265787.
Article22. Maynard SE, Venkatesha S, Thadhani R, Karumanchi SA. Soluble Fms-like tyrosine kinase 1 and endothelial dysfunction in the pathogenesis of preeclampsia. Pediatr Res. 2005; 57:1R–7R. PMID: 15557110.
Article23. Gilbert JS, Banek CT, Bauer AJ, Gingery A, Needham K. Exercise training attenuates placental ischemia-induced hypertension and angiogenic imbalance in the rat. Hypertension. 2012; 60:1545–1551. PMID: 23090773.
Article24. Arun N, Singh DP. Punica granatum: a review on pharmacological and therapeutic properties. Int J Pharm Sci Res. 2012; 2:1240–1245.25. Das S, Barman S. Antidiabetic and antihyperlipidemic effects of ethanolic extract of leaves of Punica granatum in alloxan-induced non-insulin-dependent diabetes mellitus albino rats. Indian J Pharmacol. 2012; 44:219–224. PMID: 22529479.
Article26. Viswanatha GL, Venkataranganna MV, Prasad NB, Ashok G. Evaluation of anti-epileptic activity of leaf extracts of Punica granatum on experimental models of epilepsy in mice. J Intercult Ethnopharmacol. 2016; 5:415–421. PMID: 27757273.
Article27. Dikmen M, Ozturk N, Ozturk Y. The antioxidant potency of Punica granatum L. Fruit peel reduces cell proliferation and induces apoptosis on breast cancer. J Med Food. 2011; 14:1638–1646. PMID: 21861726.28. Sineh Sepehr K, Baradaran B, Mazandarani M, Khori V, Shahneh FZ. Studies on the cytotoxic activities of punica granatum L. var. spinosa (Apple Punice) extract on prostate cell line by induction of apoptosis. ISRN Pharm. 2012; 2012:547942. PMID: 23320197.
Article29. Chen B, Longtine MS, Nelson DM. Punicalagin, a polyphenol in pomegranate juice, downregulates p53 and attenuates hypoxia-induced apoptosis in cultured human placental syncytiotrophoblasts. Am J Physiol Endocrinol Metab. 2013; 305:E1274–E1280. PMID: 24085032.
Article30. de Nigris F, Williams-Ignarro S, Botti C, Sica V, Ignarro LJ, Napoli C. Pomegranate juice reduces oxidized low-density lipoprotein downregulation of endothelial nitric oxide synthase in human coronary endothelial cells. Nitric Oxide. 2006; 15:259–263. PMID: 16413211.
Article31. BenSaad LA, Kim KH, Quah CC, Kim WR, Shahimi M. Antiinflammatory potential of ellagic acid, gallic acid and punicalagin A&B isolated from Punica granatum. BMC Complement Altern Med. 2017; 17:47. PMID: 28088220.
Article32. National Research Council (U.S.). Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Institute for Laboratory Animal Research. Guide for the care and use of laboratory animals. 8th ed. Washington, D.C.: National Academy Press;2011.33. Kemse NG, Kale AA, Joshi SR. A combined supplementation of omega-3 fatty acids and micronutrients (folic acid, vitamin B12) reduces oxidative stress markers in a rat model of pregnancy induced hypertension. PLoS One. 2014; 9:e111902. PMID: 25405347.
Article34. Kemse NG, Kale AA, Joshi SR. Supplementation of maternal omega-3 fatty acids to pregnancy induced hypertension Wistar rats improves IL10 and VEGF levels. Prostaglandins Leukot Essent Fatty Acids. 2016; 104:25–32. PMID: 26802939.
Article35. Banek CT, Bauer AJ, Gingery A, Gilbert JS. Timing of ischemic insult alters fetal growth trajectory, maternal angiogenic balance, and markers of renal oxidative stress in the pregnant rat. Am J Physiol Regul Integr Comp Physiol. 2012; 303:R658–R664. PMID: 22832532.
Article36. Galbiati S, Inversetti A, Causarano V, Stenirri S, Soriani N, Ambrosi A, Valsecchi L, Candiani M, Cremonesi L, Ferrari M, Smid M. HIF1A and MIF as potential predictive mRNA biomarkers of preeclampsia: a longitudinal prospective study in high risk population. Clin Chem Lab Med. 2015; 53:1339–1347. PMID: 25460285.
Article37. Roberts JM, Redman CW. Pre-eclampsia: more than pregnancy-induced hypertension. Lancet. 1993; 341:1447–1451. PMID: 8099148.
Article38. Anggård E. Nitric oxide: mediator, murderer, and medicine. Lancet. 1994; 343:1199–1206. PMID: 7909873.39. Molnár M, Hertelendy F. N omega-nitro-L-arginine, an inhibitor of nitric oxide synthesis, increases blood pressure in rats and reverses the pregnancy-induced refractoriness to vasopressor agents. Am J Obstet Gynecol. 1992; 166:1560–1567. PMID: 1595813.40. Yallampalli C, Garfield RE. Inhibition of nitric oxide synthesis in rats during pregnancy produces signs similar to those of preeclampsia. Am J Obstet Gynecol. 1993; 169:1316–1320. PMID: 8238200.
Article41. Vallance P, Chan N. Endothelial function and nitric oxide: clinical relevance. Heart. 2001; 85:342–350. PMID: 11179281.42. Conrad KP, Joffe GM, Kruszyna H, Kruszyna R, Rochelle LG, Smith RP, Chavez JE, Mosher MD. Identification of increased nitric oxide biosynthesis during pregnancy in rats. FASEB J. 1993; 7:566–571. PMID: 7682524.
Article43. Choi JW, Im MW, Pai SH. Nitric oxide production increases during normal pregnancy and decreases in preeclampsia. Ann Clin Lab Sci. 2002; 32:257–263. PMID: 12175088.44. Seligman SP, Buyon JP, Clancy RM, Young BK, Abramson SB. The role of nitric oxide in the pathogenesis of preeclampsia. Am J Obstet Gynecol. 1994; 171:944–948. PMID: 7943106.
Article45. Poudel R, Stanley JL, Rueda-Clausen CF, Andersson IJ, Sibley CP, Davidge ST, Baker PN. Effects of resveratrol in pregnancy using murine models with reduced blood supply to the uterus. PLoS One. 2013; 8:e64401. PMID: 23667712.
Article46. Vega CC, Reyes-Castro LA, Rodríguez-González GL, Bautista CJ, Vázquez-Martínez M, Larrea F, Chamorro-Cevallos GA, Nathanielsz PW, Zambrano E. Resveratrol partially prevents oxidative stress and metabolic dysfunction in pregnant rats fed a low protein diet and their offspring. J Physiol. 2016; 594:1483–1499. PMID: 26662841.
Article47. Xiao S, Zhang M, Liang Y, Wang D. Celastrol synergizes with oral nifedipine to attenuate hypertension in preeclampsia: a randomized, placebo-controlled, and double blinded trial. J Am Soc Hypertens. 2017; 11:598–603. PMID: 28757108.
Article48. Shi DD, Guo JJ, Zhou L, Wang N. Epigallocatechin gallate enhances treatment efficacy of oral nifedipine against pregnancy-induced severe pre-eclampsia: a double-blind, randomized and placebo-controlled clinical study. J Clin Pharm Ther. 2018; 43:21–25. PMID: 28726273.
Article49. Draganovic D, Lucic N, Jojic D. Oxidative stress marker and pregnancy induced hypertension. Med Arch. 2016; 70:437–440. PMID: 28210016.
Article50. Tanir HM, Sener T, Inal M, Akyuz F, Uzuner K, Sivri E. Effect of quercetine and glutathione on the level of superoxide dismutase, catalase, malonyldialdehyde, blood pressure and neonatal outcome in a rat model of pre-eclampsia induced by NG-nitro-L-arginine-methyl ester. Eur J Obstet Gynecol Reprod Biol. 2005; 118:190–195. PMID: 15653201.
Article51. Mehendale S, Kilari A, Dangat K, Taralekar V, Mahadik S, Joshi S. Fatty acids, antioxidants, and oxidative stress in pre-eclampsia. Int J Gynaecol Obstet. 2008; 100:234–238. PMID: 17977540.
Article52. Gohil JT, Patel PK, Gupta P. Evaluation of oxidative stress and antioxidant defence in subjects of preeclampsia. J Obstet Gynaecol India. 2011; 61:638–640. PMID: 23204680.
Article53. Yang X, Guo L, Sun X, Chen X, Tong X. Protective effects of hydrogen-rich saline in preeclampsia rat model. Placenta. 2011; 32:681–686. PMID: 21764125.
Article54. Kulkarni A, Mehendale S, Pisal H, Kilari A, Dangat K, Salunkhe S, Taralekar V, Joshi S. Association of omega-3 fatty acids and homocysteine concentrations in pre-eclampsia. Clin Nutr. 2011; 30:60–64. PMID: 20719412.
Article55. Pimentel AM, Pereira NR, Costa CA, Mann GE, Cordeiro VS, de Moura RS, Brunini TM, Mendes-Ribeiro AC, Resende AC. L-arginine-nitric oxide pathway and oxidative stress in plasma and platelets of patients with pre-eclampsia. Hypertens Res. 2013; 36:783–788. PMID: 23575380.
Article56. Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006; 160:1–40. PMID: 16430879.
Article57. Hopkins MH, Fedirko V, Jones DP, Terry PD, Bostick RM. Antioxidant micronutrients and biomarkers of oxidative stress and inflammation in colorectal adenoma patients: results from a randomized, controlled clinical trial. Cancer Epidemiol Biomarkers Prev. 2010; 19:850–858. PMID: 20200432.
Article58. Jones ML, Mark PJ, Waddell BJ. Maternal dietary omega-3 fatty acids and placental function. Reproduction. 2014; 147:R143–R152. PMID: 24451224.
Article59. Jones ML, Mark PJ, Mori TA, Keelan JA, Waddell BJ. Maternal dietary omega-3 fatty acid supplementation reduces placental oxidative stress and increases fetal and placental growth in the rat. Biol Reprod. 2013; 88:37. PMID: 23269667.
Article60. Chappell LC, Seed PT, Briley AL, Kelly FJ, Lee R, Hunt BJ, Parmar K, Bewley SJ, Shennan AH, Steer PJ, Poston L. Effect of antioxidants on the occurrence of pre-eclampsia in women at increased risk: a randomised trial. Lancet. 1999; 354:810–816. PMID: 10485722.
Article61. Chappell LC, Seed PT, Kelly FJ, Briley A, Hunt BJ, Charnock-Jones DS, Mallet A, Poston L. Vitamin C and E supplementation in women at risk of preeclampsia is associated with changes in indices of oxidative stress and placental function. Am J Obstet Gynecol. 2002; 187:777–784. PMID: 12237663.
Article62. Maynard SE, Karumanchi SA. Angiogenic factors and preeclampsia. Semin Nephrol. 2011; 31:33–46. PMID: 21266263.
Article63. Verlohren S, Stepan H, Dechend R. Angiogenic growth factors in the diagnosis and prediction of pre-eclampsia. Clin Sci (Lond). 2012; 122:43–52. PMID: 21929511.
Article64. Llurba E, Crispi F, Verlohren S. Update on the pathophysiological implications and clinical role of angiogenic factors in pregnancy. Fetal Diagn Ther. 2015; 37:81–92. PMID: 25659427.
Article65. Pavlov N, Frendo JL, Guibourdenche J, Degrelle SA, Evain-Brion D, Badet J. Angiogenin expression during early human placental development; association with blood vessel formation. Biomed Res Int. 2014; 2014:781632. PMID: 25093183.
Article66. Santos TC, Oliveira MF, Papa PC, Dantzer V, Miglino MA. VEGF system expression by immunohistochemistry and real-time RT-PCR study on collared peccary placenta. Theriogenology. 2014; 82:834–843. PMID: 25087046.
Article67. Anderson CM, Ralph JL, Ohm JE. Differential DNA methylation in placental and maternal angiogenic genes is not altered in preeclampsia. Pregnancy Hypertens. 2015; 5:45–46.68. Sahay AS, Sundrani DP, Wagh GN, Mehendale SS, Joshi SR. Regional differences in the placental levels of oxidative stress markers in pre-eclampsia. Int J Gynaecol Obstet. 2015; 129:213–218. PMID: 25813884.
Article69. Sundrani DP, Reddy US, Joshi AA, Mehendale SS, Chavan-Gautam PM, Hardikar AA, Chandak GR, Joshi SR. Differential placental methylation and expression of VEGF, FLT-1 and KDR genes in human term and preterm preeclampsia. Clin Epigenetics. 2013; 5:6. PMID: 23621880.
Article70. Sahay AS, Patil VV, Sundrani DP, Joshi AA, Wagh GN, Gupte SA, Joshi SR. A longitudinal study of circulating angiogenic and antiangiogenic factors and AT1-AA levels in preeclampsia. Hypertens Res. 2014; 37:753–758. PMID: 24718301.
Article71. Cudmore MJ, Ramma W, Cai M, Fujisawa T, Ahmad S, Al-Ani B, Ahmed A. Resveratrol inhibits the release of soluble fms-like tyrosine kinase (sFlt-1) from human placenta. Am J Obstet Gynecol. 2012; 206:253.e10–253.e15.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Induction of Angiogenic Cytokines in Cultured RPE by Oxidative Stress
- Phelligridin D maintains the function of periodontal ligament cells through autophagy in glucose-induced oxidative stress
- MLKL Inhibitor Reduces Oxidative Stress, Inflammation, and Dopaminergic Neuronal Cell Death in MPTP-Induced Parkinson’s Disease Mouse Model
- Oxidatvive Stress in Rat Model of Preeclampsia and Clinical Correlates
- Epilepsy and Oxidative Stress