J Breast Cancer.
2016 Sep;19(3):261-267. 10.4048/jbc.2016.19.3.261.
Histomorphological Factors Predicting the Response to Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer
- Affiliations
-
- 1Department of Pathology, Myongji Hospital, Goyang, Korea.
- 2Department of Pathology, Jeju National University Hospital, Jeju, Korea.
- 3Department of Pathology, Bucheon St. Mary's Hospital, The Catholic University of Korea College of Medicine, Bucheon, Korea.
- 4Department of Pathology, Seoul National University Hospital, Seoul, Korea. karlnash@naver.com
- KMID: 2413950
- DOI: http://doi.org/10.4048/jbc.2016.19.3.261
Abstract
- PURPOSE
There is no standard targeted therapy for the treatment of triple-negative breast cancer (TNBC). Therefore, its management heavily depends on adjuvant chemotherapy. Using core needle biopsy, this study evaluated the histological factors of TNBC predicting the response to chemotherapy.
METHODS
One hundred forty-three TNBC patients who received single-regimen neoadjuvant chemotherapy (NAC) with the combination of doxorubicin, cyclophosphamide, and docetaxel were enrolled. The core needle biopsy specimens acquired before NAC were used to analyze the clinicopathologic variables and overall performance of the predictive model for therapeutic response.
RESULTS
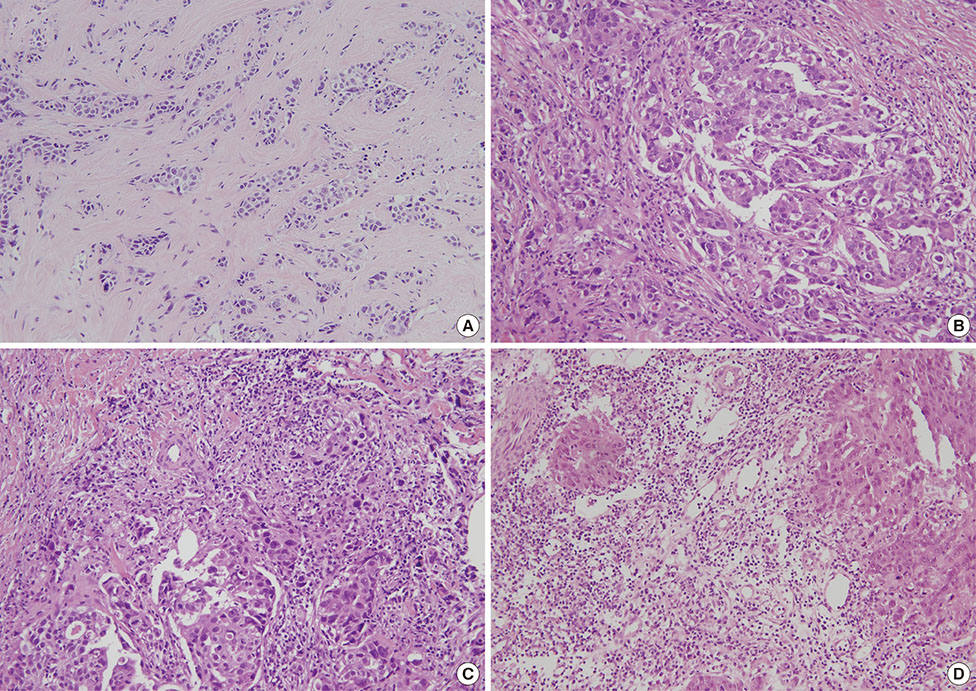
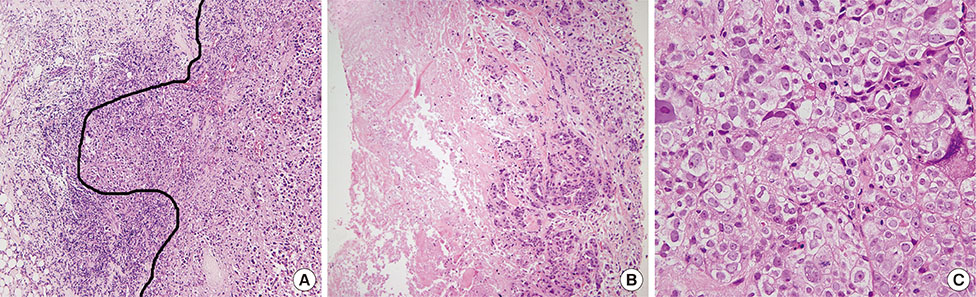
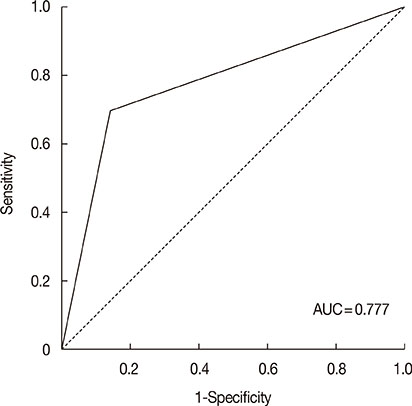
Independent predictors of pathologic complete response after NAC were found to be higher number of tumor infiltrating lymphocytes (p=0.007), absence of clear cytoplasm (p=0.008), low necrosis (p=0.018), and high histologic grade (p=0.039). In the receiver operating characteristics curve analysis, the area under curve for the combination of these four variables was 0.777.
CONCLUSION
The present study demonstrated that a predictive model using the above four variables can predict therapeutic response to single-regimen NAC with the combination of doxorubicin, cyclophosphamide, and docetaxel in TNBC. Therefore, adding these morphologic variables to clinical and genomic signatures might enhance the ability to predict the therapeutic response to NAC in TNBC.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Comment on “Histomorphological Factors Predicting the Response to Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer”
Kadri Altundag
J Breast Cancer. 2017;20(1):114-115. doi: 10.4048/jbc.2017.20.1.114.
Reference
-
1. von Minckwitz G, Raab G, Caputo A, Schütte M, Hilfrich J, Blohmer JU, et al. Doxorubicin with cyclophosphamide followed by docetaxel every 21 days compared with doxorubicin and docetaxel every 14 days as preoperative treatment in operable breast cancer: the GEPARDUO study of the German Breast Group. J Clin Oncol. 2005; 23:2676–2685.
Article2. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Clarke M, Coates AS, Darby SC, Davies C, Gelber RD, et al. Adjuvant chemotherapy in oestrogen-receptor-poor breast cancer: patient-level meta-analysis of randomised trials. Lancet. 2008; 371:29–40.
Article3. Berry DA, Cirrincione C, Henderson IC, Citron ML, Budman DR, Goldstein LJ, et al. Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA. 2006; 295:1658–1667.
Article4. Bear HD, Tang G, Rastogi P, Geyer CE Jr, Robidoux A, Atkins JN, et al. Bevacizumab added to neoadjuvant chemotherapy for breast cancer. N Engl J Med. 2012; 366:310–320.
Article5. Torrisi R, Balduzzi A, Ghisini R, Rocca A, Bottiglieri L, Giovanardi F, et al. Tailored preoperative treatment of locally advanced triple negative (hormone receptor negative and HER2 negative) breast cancer with epirubicin, cisplatin, and infusional fluorouracil followed by weekly paclitaxel. Cancer Chemother Pharmacol. 2008; 62:667–672.
Article6. Nabholtz JM, Abrial C, Mouret-Reynier MA, Dauplat MM, Weber B, Gligorov J, et al. Multicentric neoadjuvant phase II study of panitumumab combined with an anthracycline/taxane-based chemotherapy in operable triple-negative breast cancer: identification of biologically defined signatures predicting treatment impact. Ann Oncol. 2014; 25:1570–1577.
Article7. Yoo SH, Park IA, Chung YR, Kim H, Lee K, Noh DY, et al. A histomorphologic predictive model for axillary lymph node metastasis in preoperative breast cancer core needle biopsy according to intrinsic subtypes. Hum Pathol. 2015; 46:246–254.
Article8. Ryu HS, Kim WH, Ahn S, Kim DW, Kang SB, Park HJ, et al. Combined morphologic and molecular classification for predicting lymph node metastasis in early-stage colorectal adenocarcinoma. Ann Surg Oncol. 2014; 21:1809–1816.
Article9. Jung YY, Lee CH, Park SY, Park HJ, Min HS, Won JK, et al. Characteristic tumor growth patterns as novel histomorphologic predictors for lymph node metastasis in papillary thyroid carcinoma. Hum Pathol. 2013; 44:2620–2627.
Article10. Chung YJ, Lee JS, Park SY, Park HJ, Cho BY, Park SJ, et al. Histomorphological factors in the risk prediction of lymph node metastasis in papillary thyroid carcinoma. Histopathology. 2013; 62:578–588.
Article11. Acs G, Paragh G, Chuang ST, Laronga C, Zhang PJ. The presence of micropapillary features and retraction artifact in core needle biopsy material predicts lymph node metastasis in breast carcinoma. Am J Surg Pathol. 2009; 33:202–210.
Article12. Wang J, Buchholz TA, Middleton LP, Allred DC, Tucker SL, Kuerer HM, et al. Assessment of histologic features and expression of biomarkers in predicting pathologic response to anthracycline-based neoadjuvant chemotherapy in patients with breast carcinoma. Cancer. 2002; 94:3107–3114.
Article13. Pu RT, Schott AF, Sturtz DE, Griffith KA, Kleer CG. Pathologic features of breast cancer associated with complete response to neoadjuvant chemotherapy: importance of tumor necrosis. Am J Surg Pathol. 2005; 29:354–358.
Article14. Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014; 384:164–172.
Article15. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991; 19:403–410.
Article16. Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015; 26:259–271.
Article17. Schalper KA, Velcheti V, Carvajal D, Wimberly H, Brown J, Pusztai L, et al. In situ tumor PD-L1 mRNA expression is associated with increased TILs and better outcome in breast carcinomas. Clin Cancer Res. 2014; 20:2773–2782.
Article18. Acs G, Dumoff KL, Solin LJ, Pasha T, Xu X, Zhang PJ. Extensive retraction artifact correlates with lymphatic invasion and nodal metastasis and predicts poor outcome in early stage breast carcinoma. Am J Surg Pathol. 2007; 31:129–140.
Article19. Travis WD. Advances in neuroendocrine lung tumors. Ann Oncol. 2010; 21:Suppl 7. vii65–vii71.
Article20. Kumar V. AK Abbas. In : Aster JC, editor. Robbins and Cotran Pathologic Basis of Disease. 9th ed. Philadelphia: Elsevier Saunders;2015.21. Hatzis C, Pusztai L, Valero V, Booser DJ, Esserman L, Lluch A, et al. A genomic predictor of response and survival following taxane-anthracycline chemotherapy for invasive breast cancer. JAMA. 2011; 305:1873–1881.
Article22. Alba E, Chacon JI, Lluch A, Anton A, Estevez L, Cirauqui B, et al. A randomized phase II trial of platinum salts in basal-like breast cancer patients in the neoadjuvant setting: results from the GEICAM/2006-03, multicenter study. Breast Cancer Res Treat. 2012; 136:487–493.
Article23. Kim KI, Lee KH, Kim TR, Chun YS, Lee TH, Park HK. Ki-67 as a predictor of response to neoadjuvant chemotherapy in breast cancer patients. J Breast Cancer. 2014; 17:40–46.
Article24. Zhang G, Xie W, Liu Z, Lin C, Piao Y, Xu L, et al. Prognostic function of Ki-67 for pathological complete response rate of neoadjuvant chemotherapy in triple-negative breast cancer. Tumori. 2014; 100:136–142.
Article25. Rubovszky G, Horváth Z, Tóth E, Láng I, Kásler M. Significance of histomorphology of early triple-negative breast cancer. Pathol Oncol Res. 2012; 18:823–831.
Article26. Ueda S, Roblyer D, Cerussi A, Durkin A, Leproux A, Santoro Y, et al. Baseline tumor oxygen saturation correlates with a pathologic complete response in breast cancer patients undergoing neoadjuvant chemotherapy. Cancer Res. 2012; 72:4318–4328.
Article27. Patel T, Gupta A, Shah M. Pathological predictive factors for tumor response in locally advanced breast carcinomas treated with anthracyclin-based neoadjuvant chemotherapy. J Cancer Res Ther. 2013; 9:245–249.
Article28. Itamochi H, Kigawa J, Terakawa N. Mechanisms of chemoresistance and poor prognosis in ovarian clear cell carcinoma. Cancer Sci. 2008; 99:653–658.
Article29. Hodge JW, Garnett CT, Farsaci B, Palena C, Tsang KY, Ferrone S, et al. Chemotherapy-induced immunogenic modulation of tumor cells enhances killing by cytotoxic T lymphocytes and is distinct from immunogenic cell death. Int J Cancer. 2013; 133:624–636.
Article30. Alizadeh D, Trad M, Hanke NT, Larmonier CB, Janikashvili N, Bonnotte B, et al. Doxorubicin eliminates myeloid-derived suppressor cells and enhances the efficacy of adoptive T-cell transfer in breast cancer. Cancer Res. 2014; 74:104–118.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comment on “Histomorphological Factors Predicting the Response to Neoadjuvant Chemotherapy in Triple-Negative Breast Cancerâ€
- Ki-67 as a Predictor of Response to Neoadjuvant Chemotherapy in Breast Cancer Patients
- Pathologic Findings of Residual Tumor according to the Response Rate after Neoadjuvant Chemotherapy for Breast Cancer
- Differences in prognosis by p53 expression after neoadjuvant chemotherapy in triple-negative breast cancer
- Bilateral Triple Negative Invasive Ductal Breast Carcinoma in a BRCA1 Mutation Carrier with Discrepant Pathologic Response to Neoadjuvant Chemotherapy