Korean J Radiol.
2018 Aug;19(4):560-567. 10.3348/kjr.2018.19.4.560.
Comparison of Chronologic Change in the Size and Contrast-Enhancement of Ablation Zones on CT Images after Irreversible Electroporation and Radiofrequency Ablation
- Affiliations
-
- 1Department of Diagnostic and Interventional Radiology, Aachen University Hospital, RWTH Aachen University, Aachen 52074, Germany. isfort@ukaachen.de
- 2Institute of Medical Statistics, Aachen University Hospital, RWTH Aachen University, Aachen 52074, Germany.
- KMID: 2413685
- DOI: http://doi.org/10.3348/kjr.2018.19.4.560
Abstract
OBJECTIVE
To compare short-, mid-, and long-term follow-up ablation zone volume alterations as well as imaging features on contrast-enhanced computed tomography (CT) after irreversible electroporation (IRE) of primary and secondary liver tumors with findings subsequent to radiofrequency ablation (RFA).
MATERIALS AND METHODS
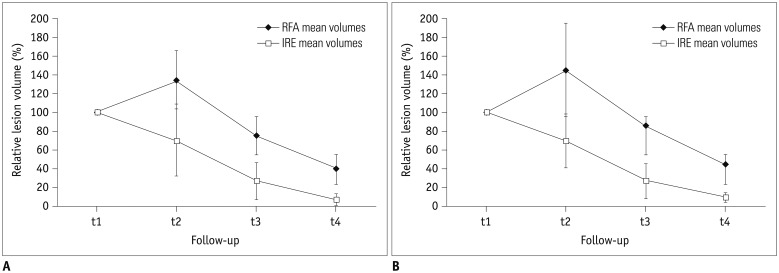
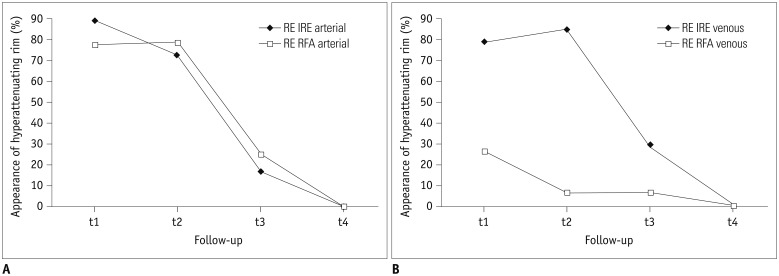
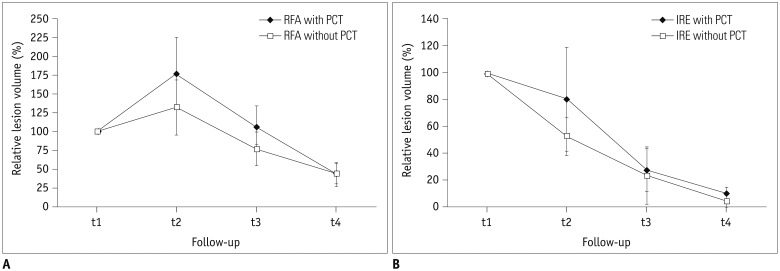
Volume assessment of 39 ablation zones (19 RFA, 20 IRE) after intervention was performed at four time intervals (day 0 [t1; n = 39], day 1-7 [t2; n = 25], day 8-55 [t3; n = 28], after day 55 [t4; n = 23]) on dual-phase CT. Analysis of peripheral rim enhancement was conducted. Lesion's volume decrease relative to the volume at t1 was calculated and statistically analyzed with respect to patient's sex, age, ablation modality (IRE/RFA), and history of platinum-based chemotherapy (PCT).
RESULTS
No influence of patient's sex or age on ablation volume was detected. The decrease in ablation zones' volume was significantly larger (p < 0.05 for all time intervals) after IRE (arterial phase, 7.5%; venous phase, 9.7% of initial volume) compared to RFA (arterial phase, 39.6%; venous phase, 45.3% of initial volume). After RFA, significantly smaller decreases in the ablation volumes, in general, were detected in patients treated with PCT in their history (p = 0.004), which was not detected after IRE (p = 0.288). In the arterial phase, peripheral rim enhancement was frequently detected after both IRE and RFA. In the venous phase, rim-enhancement was depicted significantly more often following IRE at t1 and t2 (pt1 = 0.003, pt2 < 0.001).
CONCLUSION
As per our analysis, ablation zone volume decreased significantly in a more rapid and more profound manner after IRE. Lesion's remodeling after RFA but not IRE seems to be influenced by PCT, possibly due to the type of cell death induced by the different ablation modalities.
Keyword
MeSH Terms
Figure
Reference
-
1. Davalos RV, Mir IL, Rubinsky B. Tissue ablation with irreversible electroporation. Ann Biomed Eng. 2005; 33:223–231. PMID: 15771276.
Article2. Lee EW, Chen C, Prieto VE, Dry SM, Loh CT, Kee ST. Advanced hepatic ablation technique for creating complete cell death: irreversible electroporation. Radiology. 2010; 255:426–433. PMID: 20413755.
Article3. Maor E, Ivorra A, Leor J, Rubinsky B. The effect of irreversible electroporation on blood vessels. Technol Cancer Res Treat. 2007; 6:307–312. PMID: 17668938.
Article4. Rubinsky B, Onik G, Mikus P. Irreversible electroporation: a new ablation modality--clinical implications. Technol Cancer Res Treat. 2007; 6:37–48. PMID: 17241099.5. Chung DJ, Sung K, Osuagwu FC, Wu HH, Lassman C, Lu DS. Contrast enhancement patterns after irreversible electroporation: experimental study of CT perfusion correlated to histopathology in normal porcine liver. J Vasc Interv Radiol. 2016; 27:104–111. PMID: 26547121.
Article6. Dollinger M, Jung EM, Beyer L, Niessen C, Scheer F, Müller-Wille R, et al. Irreversible electroporation ablation of malignant hepatic tumors: subacute and follow-up CT appearance of ablation zones. J Vasc Interv Radiol. 2014; 25:1589–1594. PMID: 25156648.
Article7. Li S, Zeng Q, Zhong R, Mao S, Shen L, Wu P. [Liver regeneration after radiofrequency ablation versus irreversible electroporation]. Zhonghua Yi Xue Za Zhi. 2015; 95:66–68. PMID: 25876814.8. Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin JC, Pujol S, et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging. 2012; 30:1323–1341. PMID: 22770690.
Article9. Cheng RG, Bhattacharya R, Yeh MM, Padia SA. Irreversible electroporation can effectively ablate hepatocellular carcinoma to complete pathologic necrosis. J Vasc Interv Radiol. 2015; 26:1184–1188. PMID: 26119204.
Article10. Cheung W, Kavnoudias H, Roberts S, Szkandera B, Kemp W, Thomson KR. Irreversible electroporation for unresectable hepatocellular carcinoma: initial experience and review of safety and outcomes. Technol Cancer Res Treat. 2013; 12:233–241. PMID: 23369152.
Article11. Scheffer HJ, Nielsen K, de Jong MC, van Tilborg AA, Vieveen JM, Bouwman AR, et al. Irreversible electroporation for nonthermal tumor ablation in the clinical setting: a systematic review of safety and efficacy. J Vasc Interv Radiol. 2014; 25:997–1011. quiz 1011. PMID: 24656178.
Article12. Thomson KR, Cheung W, Ellis SJ, Federman D, Kavnoudias H, Loader-Oliver D, et al. Investigation of the safety of irreversible electroporation in humans. J Vasc Interv Radiol. 2011; 22:611–621. PMID: 21439847.
Article13. Niessen C, Jung EM, Wohlgemuth WA, Trabold B, Haimerl M, Schreyer A, et al. Irreversible electroporation of a hepatocellular carcinoma lesion adjacent to a transjugular intrahepatic portosystemic shunt stent graft. Korean J Radiol. 2013; 14:797–800. PMID: 24043975.
Article14. Lee YJ, Lu DS, Osuagwu F, Lassman C. Irreversible electroporation in porcine liver: acute computed tomography appearance of ablation zone with histopathologic correlation. J Comput Assist Tomogr. 2013; 37:154–158. PMID: 23493202.15. Goldberg SN, Kamel IR, Kruskal JB, Reynolds K, Monsky WL, Stuart KE, et al. Radiofrequency ablation of hepatic tumors: increased tumor destruction with adjuvant liposomal doxorubicin therapy. AJR Am J Roentgenol. 2002; 179:93–101. PMID: 12076912.
Article16. Keil S, Bruners P, Schiffl K, Sedlmair M, Mühlenbruch G, Günther RW, et al. Radiofrequency ablation of liver metastases-software-assisted evaluation of the ablation zone in MDCT: tumor-free follow-up versus local recurrent disease. Cardiovasc Intervent Radiol. 2010; 33:297–306. PMID: 19688366.
Article17. Lim HK, Choi D, Lee WJ, Kim SH, Lee SJ, Jang HJ, et al. Hepatocellular carcinoma treated with percutaneous radio-frequency ablation: evaluation with follow-up multiphase helical CT. Radiology. 2001; 221:447–454. PMID: 11687689.
Article18. Siperstein A, Garland A, Engle K, Rogers S, Berber E, Foroutani A, et al. Local recurrence after laparoscopic radiofrequency thermal ablation of hepatic tumors. Ann Surg Oncol. 2000; 7:106–113. PMID: 10761788.
Article19. Goldberg SN, Gazelle GS, Compton CC, Mueller PR, Tanabe KK. Treatment of intrahepatic malignancy with radiofrequency ablation: radiologic-pathologic correlation. Cancer. 2000; 88:2452–2463. PMID: 10861420.20. Morimoto M, Sugimori K, Shirato K, Kokawa A, Tomita N, Saito T, et al. Treatment of hepatocellular carcinoma with radiofrequency ablation: radiologic-histologic correlation during follow-up periods. Hepatology. 2002; 35:1467–1475. PMID: 12029632.
Article21. Bertrand J, Caillol F, Borentain P, Raoul JL, Heyries L, Bories E, et al. Percutaneous hepatic radiofrequency for hepatocellular carcinoma: results and outcome of 46 patients. Hepat Med. 2015; 7:21–27. PMID: 26056497.
Article22. Park MH, Rhim H, Kim YS, Choi D, Lim HK, Lee WJ. Spectrum of CT findings after radiofrequency ablation of hepatic tumors. Radiographics. 2008; 28:379–390. discussion 390-392. PMID: 18349446.
Article23. Kim SD, Yoon SG, Sung GT. Radiofrequency ablation of renal tumors: four-year follow-up results in 47 patients. Korean J Radiol. 2012; 13:625–633. PMID: 22977331.
Article24. Kim HB, Sung CK, Baik KY, Moon KW, Kim HS, Yi JH, et al. Changes of apoptosis in tumor tissues with time after irreversible electroporation. Biochem Biophys Res Commun. 2013; 435:651–656. PMID: 23688425.
Article25. Golberg A, Bruinsma BG, Jaramillo M, Yarmush ML, Uygun BE. Rat liver regeneration following ablation with irreversible electroporation. PeerJ. 2016; 4:e1571. PMID: 26819842.
Article26. Martins NM, Santos NA, Curti C, Bianchi ML, Santos AC. Cisplatin induces mitochondrial oxidative stress with resultant energetic metabolism impairment, membrane rigidification and apoptosis in rat liver. J Appl Toxicol. 2008; 28:337–344. PMID: 17604343.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Irreversible electroporation of hepatocellular carcinoma: the role of ultrasonography
- Is electrocardiogram-gated irreversible electroporation still effective in liver ablation? A validation study in swine liver irreversible electroporation model
- Current Research Status of Irreversible Electroporation for Hollow Viscus Organ of Gastrointestinal Tract
- Irreversible Electroporation: A Novel Image-Guided Cancer Therapy
- Irreversible Electroporation of a Hepatocellular Carcinoma Lesion Adjacent to a Transjugular Intrahepatic Portosystemic Shunt Stent Graft