Int J Stem Cells.
2018 Jun;11(1):96-104. 10.15283/ijsc17049.
Optimal Xeno-free Culture Condition for Clinical Grade Stem Cells from Human Exfoliated Deciduous Teeth
- Affiliations
-
- 1Department of Orthodontics, Faculty of Dentistry, Mahidol University, Bangkok, Thailand.
- 2Department of Oral Biology, Faculty of Dentistry, Mahidol University, Bangkok, Thailand. hathaitip.sri@mahidol.ac.th
- KMID: 2413505
- DOI: http://doi.org/10.15283/ijsc17049
Abstract
- BACKGROUND AND OBJECTIVES
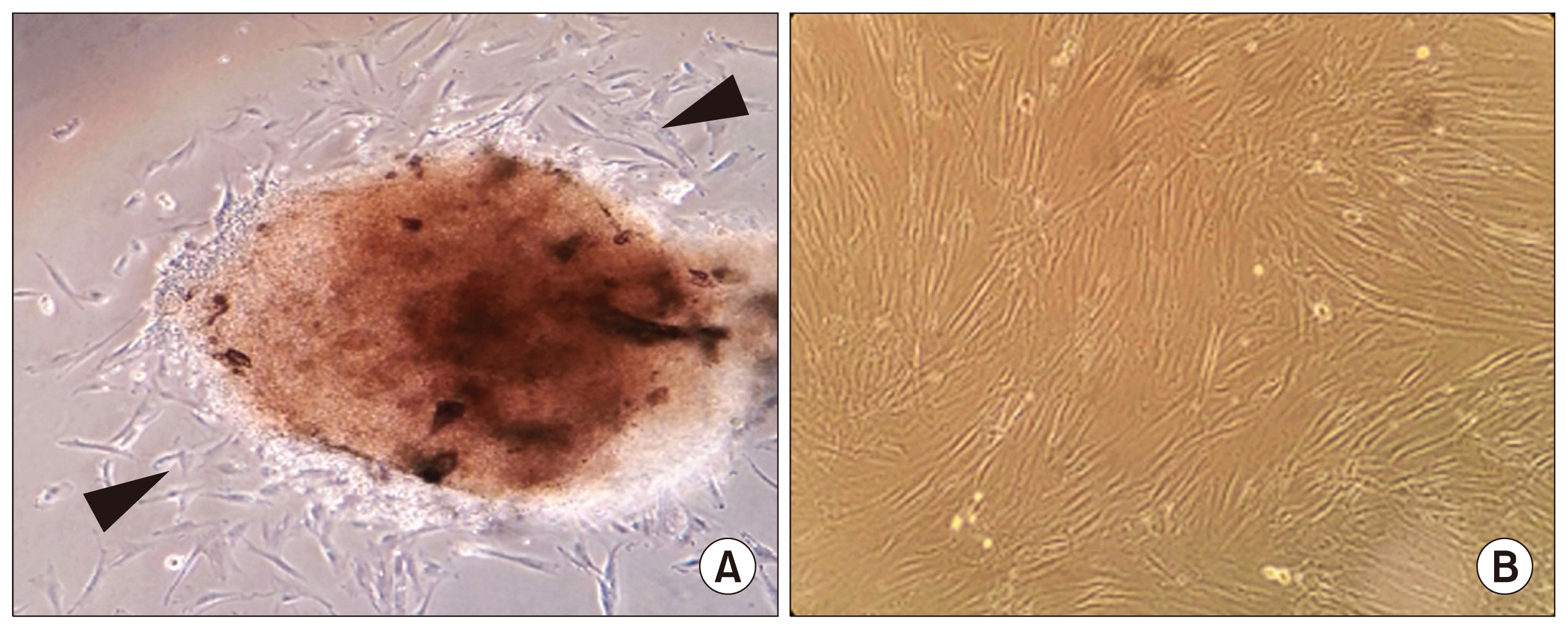
Stem cells from human exfoliated deciduous teeth (SHED) are a promising clinical resource for various tissue defects, including lumbar spondylosis, neural compression, and cleft palate. Use of media containing animal-derived serum carries potential risk of infectious diseases and unwanted immunogenicity. To increase the potential utility of SHED for clinical application, SHED was adapted to xeno-free conditions.
METHODS
Define xeno-free culture media were compared with the conventional serum containing media in the culture of SHED. Cultured SHED in different media were further characterized through proliferative capacities, cellular phenotype, and differentiation potential.
RESULTS
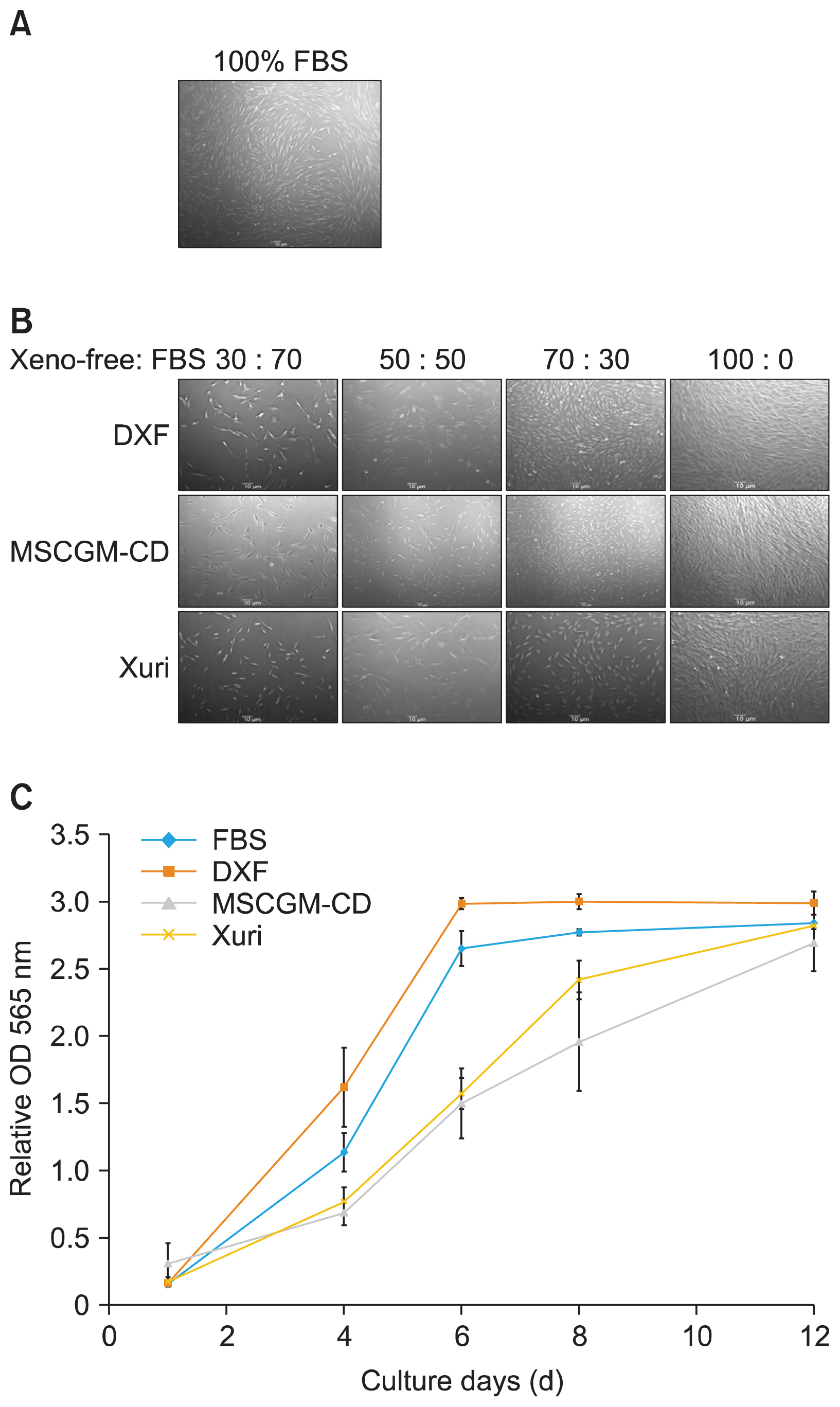
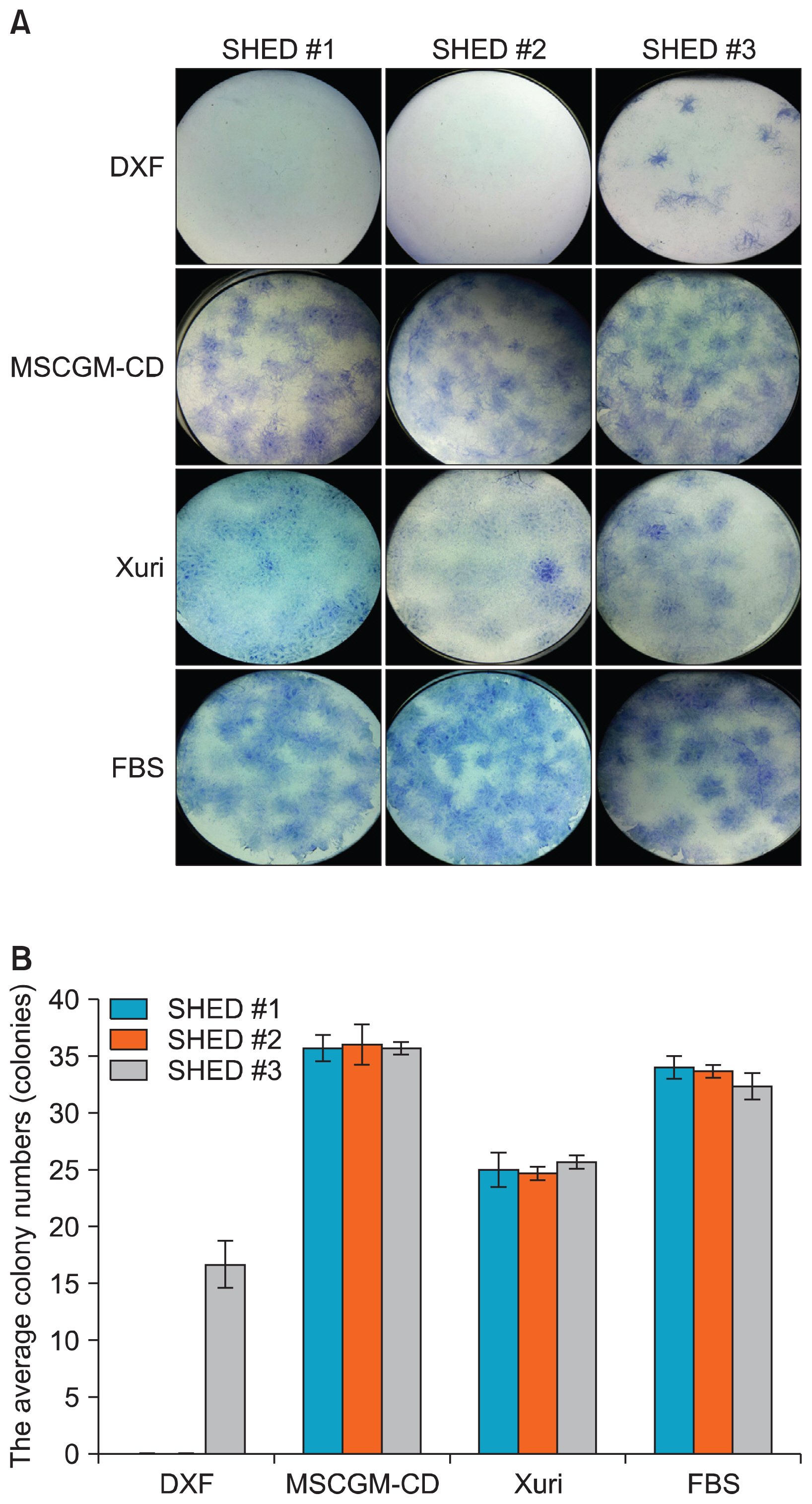
Selected xeno-free media were capable of supporting the growth of SHED. MSCGM-CD Bulletkit medium greatly increased the number and proliferate capacity of colony-forming unit-fibroblast than SHED cultured in other media. In addition, the characteristic surface markers expression and multipotent differentiation potential of SHED in the MSCGM-CD Bulletkit medium were comparable to those observed with serum-containing medium.
CONCLUSIONS
The xeno-free medium described herein has the potential to be further used for the safe expansion and to determine efficient way to produce clinical grade dental stem cells for therapeutic applications.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003; 100:5807–5812. DOI: 10.1073/pnas.0937635100. PMID: 12716973. PMCID: 156282.
Article2. Demarco FF, Conde MC, Cavalcanti BN, Casagrande L, Sakai VT, Nör JE. Dental pulp tissue engineering. Braz Dent J. 2011; 22:3–13. DOI: 10.1590/S0103-64402011000100001. PMID: 21519641. PMCID: 3736569.
Article3. Nör JE. Tooth regeneration in operative dentistry. Oper Dent. 2006; 31:633–642. DOI: 10.2341/06-000. PMID: 17153970.4. Potdar PD, Jethmalani YD. Human dental pulp stem cells: Applications in future regenerative medicine. World J Stem Cells. 2015; 7:839–851. DOI: 10.4252/wjsc.v7.i5.839. PMID: 26131314. PMCID: 4478630.
Article5. Mannello F, Tonti GA. Concise review: no breakthroughs for human mesenchymal and embryonic stem cell culture: conditioned medium, feeder layer, or feeder-free; medium with fetal calf serum, human serum, or enriched plasma; serum-free, serum replacement nonconditioned medium, or ad hoc formula? All that glitters is not gold! Stem Cells. 2007; 25:1603–1609. DOI: 10.1634/stemcells.2007-0127. PMID: 17395775.
Article6. Ankrum J, Karp JM. Mesenchymal stem cell therapy: Two steps forward, one step back. Trends Mol Med. 2010; 16:203–209. DOI: 10.1016/j.molmed.2010.02.005. PMID: 20335067. PMCID: 2881950.
Article7. Halme DG, Kessler DA. FDA regulation of stem-cell-based therapies. N Engl J Med. 2006; 355:1730–1735. DOI: 10.1056/NEJMhpr063086. PMID: 17050899.
Article8. Spees JL, Gregory CA, Singh H, Tucker HA, Peister A, Lynch PJ, Hsu SC, Smith J, Prockop DJ. Internalized antigens must be removed to prepare hypoimmunogenic mesenchymal stem cells for cell and gene therapy. Mol Ther. 2004; 9:747–756. DOI: 10.1016/j.ymthe.2004.02.012. PMID: 15120336.
Article9. Kerkis I, Caplan AI. Stem cells in dental pulp of deciduous teeth. Tissue Eng Part B Rev. 2012; 18:129–138. DOI: 10.1089/ten.teb.2011.0327. PMCID: 3311402.
Article10. Tonti GA, Mannello F. From bone marrow to therapeutic applications: different behaviour and genetic/epigenetic stability during mesenchymal stem cell expansion in autologous and foetal bovine sera? Int J Dev Biol. 2008; 52:1023–1032. DOI: 10.1387/ijdb.082725gt. PMID: 18956335.
Article11. Stute N, Holtz K, Bubenheim M, Lange C, Blake F, Zander AR. Autologous serum for isolation and expansion of human mesenchymal stem cells for clinical use. Exp Hematol. 2004; 32:1212–1225. DOI: 10.1016/j.exphem.2004.09.003. PMID: 15588946.
Article12. Zhang D, Mai Q, Li T, Huang J, Ding C, Jia M, Zhou C, Xu Y. Comparison of a xeno-free and serum-free culture system for human embryonic stem cells with conventional culture systems. Stem Cell Res Ther. 2016; 7:101. DOI: 10.1186/s13287-016-0347-7. PMID: 27474011. PMCID: 4967296.
Article13. Miyagi-Shiohira C, Kobayashi N, Saitoh I, Watanabe M, Noguchi Y, Matsushita M, Noguchi H. Evaluation of serum-free, xeno-free cryopreservation solutions for human adipose-derived mesenchymal stem cells. Cell Med. 2016; 9:15–20. DOI: 10.3727/215517916X693122.
Article14. Bieback K, Hecker A, Kocaömer A, Lannert H, Schallmoser K, Strunk D, Klüter H. Human alternatives to fetal bovine serum for the expansion of mesenchymal stromal cells from bone marrow. Stem Cells. 2009; 27:2331–2341. DOI: 10.1002/stem.139. PMID: 19544413.
Article15. Ben Azouna N, Jenhani F, Regaya Z, Berraeis L, Ben Othman T, Ducrocq E, Domenech J. Phenotypical and functional characteristics of mesenchymal stem cells from bone marrow: comparison of culture using different media supplemented with human platelet lysate or fetal bovine serum. Stem Cell Res Ther. 2012; 3:6. DOI: 10.1186/scrt97. PMID: 22333342. PMCID: 3340550.
Article16. Bieback K, Kern S, Kocaömer A, Ferlik K, Bugert P. Comparing mesenchymal stromal cells from different human tissues: bone marrow, adipose tissue and umbilical cord blood. Biomed Mater Eng. 2008; 18(1 Suppl):S71–S76. PMID: 18334717.17. Takeda-Kawaguchi T, Sugiyama K, Chikusa S, Iida K, Aoki H, Tamaoki N, Hatakeyama D, Kunisada T, Shibata T, Fusaki N, Tezuka K. Derivation of iPSCs after culture of human dental pulp cells under defined conditions. PLoS One. 2014; 9:e115392. DOI: 10.1371/journal.pone.0115392. PMID: 25521610. PMCID: 4270765.
Article18. Pelikant-Malecka I, Kaniewska-Bednarczuk E, Szrok S, Sielicka A, Sledzinski M, Orlewska C, Smolenski RT, Slominska EM. Metabolic pathway of 4-pyridone-3-carboxamide-1β-d-ribonucleoside and its effects on cellular energetics. Int J Biochem Cell Biol. 2017; 88:31–43. DOI: 10.1016/j.biocel.2017.03.012. PMID: 28323211.
Article19. Gerby S, Attebi E, Vlaski M, Ivanovic Z. A new clinical-scale serum-free xeno-free medium efficient in ex vivo amplification of mesenchymal stromal cells does not support mesenchymal stem cells. Transfusion. 2017; 57:433–439. DOI: 10.1111/trf.13902.
Article20. Colter DC, Sekiya I, Prockop DJ. Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proc Natl Acad Sci U S A. 2001; 98:7841–7845. DOI: 10.1073/pnas.141221698. PMID: 11427725. PMCID: 35429.
Article21. Ng F, Boucher S, Koh S, Sastry KS, Chase L, Lakshmipathy U, Choong C, Yang Z, Vemuri MC, Rao MS, Tanavde V. PDGF, TGF-beta, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood. 2008; 112:295–307. DOI: 10.1182/blood-2007-07-103697. PMID: 18332228.
Article22. Chase LG, Lakshmipathy U, Solchaga LA, Rao MS, Vemuri MC. A novel serum-free medium for the expansion of human mesenchymal stem cells. Stem Cell Res Ther. 2010; 1:8. DOI: 10.1186/scrt8. PMID: 20504289. PMCID: 3226302.
Article23. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006; 8:315–317. DOI: 10.1080/14653240600855905.
Article24. Hughes DE, Salter DM, Simpson R. CD44 expression in human bone: a novel marker of osteocytic differentiation. J Bone Miner Res. 1994; 9:39–44. DOI: 10.1002/jbmr.5650090106. PMID: 7512306.
Article25. Takedachi M, Oohara H, Smith BJ, Iyama M, Kobashi M, Maeda K, Long CL, Humphrey MB, Stoecker BJ, Toyosawa S, Thompson LF, Murakami S. CD73-generated adenosine promotes osteoblast differentiation. J Cell Physiol. 2012; 227:2622–2631. DOI: 10.1002/jcp.23001.
Article26. Cavaliere F, Donno C, D’Ambrosi N. Purinergic signaling: a common pathway for neural and mesenchymal stem cell maintenance and differentiation. Front Cell Neurosci. 2015; 9:211. DOI: 10.3389/fncel.2015.00211. PMID: 26082684. PMCID: 4451364.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Indirect Co-Culture of Stem Cells from Human Exfoliated Deciduous Teeth and Oral Cells in a Microfluidic Platform
- Clean-Up Human Embryonic Stem Cell Lines Using Humanized Culture Condition
- Influences of Xeno-Free Media on Mesenchymal Stem Cell Expansion for Clinical Application
- Cytotoxicity of Various Calcium Silicate-based Materials with Stem Cells from Deciduous Teeth
- Maintenance of hPSCs under Xeno-Free and Chemically Defined Culture Conditions