Allergy Asthma Immunol Res.
2017 Mar;9(2):142-151. 10.4168/aair.2017.9.2.142.
Vγ1+γδT Cells Are Correlated With Increasing Expression of Eosinophil Cationic Protein and Metalloproteinase-7 in Chronic Rhinosinusitis With Nasal Polyps Inducing the Formation of Edema
- Affiliations
-
- 1Department of Otorhinolaryngology, the Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, China. gehuazh@hotmail.com changlihong19840719@126.com
- KMID: 2413388
- DOI: http://doi.org/10.4168/aair.2017.9.2.142
Abstract
- PURPOSE
We have found that expression of γδT cells is increased in pathological mucosa of chronic rhinosinusitis with nasal polyps (CRSwNP) compared with normal nasal mucosa. This increase is correlated with the infiltration of eosinophils in CRSwNP. Here, we investigated the expression of γδT cells, inflammation and tissue remodeling factors as well as their probable relationships in different types of chronic rhinosinusitis (CRS) in China.
METHODS
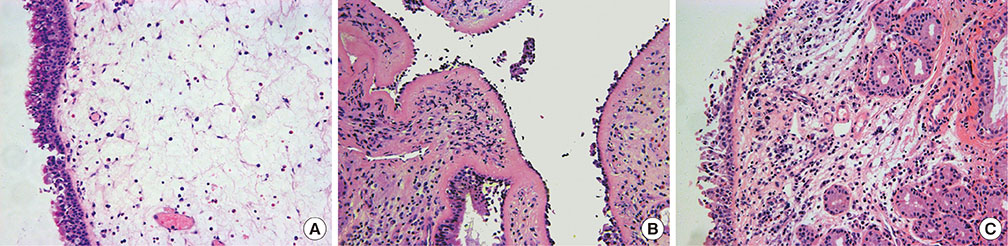
A total of 76 surgical tissue samples that included 43 CRSwNP samples (15 eosinophilic and 28 non-eosinophilic), 17 CRS samples without nasal polyps (CRSsNP), and 16 controls were obtained. Quantitative reverse transcription-polymerase chain reaction (RT-PCR) was used to measure the mRNA expression levels of Vγ1âºÎ³Î´T cells, Vγ4âºÎ³Î´T cells, eosinophil cationic protein (ECP), interleukin (IL)-8, transforming growth factor (TGF)-β2, metalloproteinase (MMP)-7, tissue inhibitor of metalloproteinase (TIMP)-4 and hypoxia-inducible factor (HIF)-1α. Enzyme linked immunosorbent assay (ELISA) was used to measure the protein level of ECP and MMP-7 in CRSwNP. The eosinophils were counted and the level of edema was analyzed with HE staining.
RESULTS
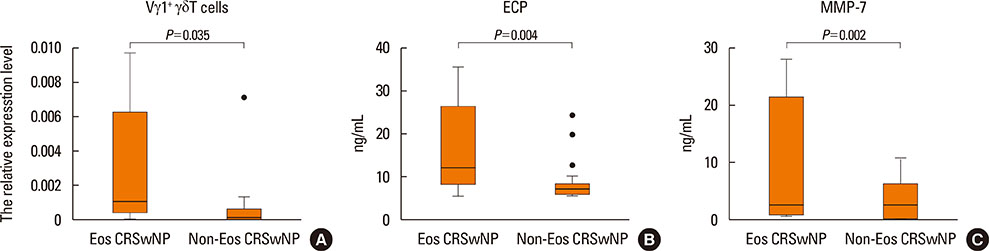
The mRNA expression levels of the Vγ1 subset, ECP and MMP-7 were significantly increased in CRSwNP with histological characteristics of eosinophilic infiltration and edema. The expression of the Vγ1 gene in CRSwNP correlated positively with the expression of both ECP and MMP-7. No significant decreases in the mRNA expression levels of TGF-β2, TIMP-4 or HIF-1α were observed in the CRSwNP samples. The expression levels of Vγ1 gene, ECP and MMP-7 were significantly increased in eosinophilic CRSwNP compared to non-eosinophilic CRSwNP.
CONCLUSIONS
Our results suggest the associations between Vγ1âºÎ³Î´T cells, ECP and MMP-7 in CRSwNP, indicating that Vγ1âºÎ³Î´T cells can induce the eosinophilic inflammation, which has a further effect on the formation of edema.
MeSH Terms
Figure
Cited by 1 articles
-
The Correlation of Tissue’s Endotype Biomarkers and Dominant Inflammation Cell in Chronic Rhinosinusitis
Iriana Maharani, Monica Intan, Dyah Indrasworo, Kenty Wantri Anita
Korean J Otorhinolaryngol-Head Neck Surg. 2024;67(3):152-158. doi: 10.3342/kjorl-hns.2023.00493.
Reference
-
1. Blackwell DL, Lucas JW, Clarke TC. Summary health statistics for U.S. adults: national health interview survey, 2012. Vital Health Stat. 2014; 10:1–161.2. Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. European position paper on rhinosinusitis and nasal polyps 2012. Rhinology. 2012; 50:Suppl 23. 1–298.3. Shi LL, Xiong P, Zhang L, Cao PP, Liao B, Lu X, et al. Features of air-way remodeling in different types of Chinese chronic rhinosinusitis are associated with inflammation patterns. Allergy. 2013; 68:101–109.4. Brenner MB, McLean J, Dialynas DP, Strominger JL, Smith JA, Owen FL, et al. Identification of a putative second T-cell receptor. Nature. 1986; 322:145–149.5. Yan X, Shichita T, Katsumata Y, Matsuhashi T, Ito H, Ito K, et al. Deleterious effect of the IL-23/IL-17A axis and gammadeltaT cells on left ventricular remodeling after myocardial infarction. J Am Heart Assoc. 2012; 1:e004408.6. Li Y, Wu Y, Zhang C, Li P, Cui W, Hao J, et al. γδT cell-derived interleukin-17A via an interleukin-1β-dependent mechanism mediates cardiac injury and fibrosis in hypertension. Hypertension. 2014; 64:305–314.7. Chen D, Luo X, Xie H, Gao Z, Fang H, Huang J. Characteristics of IL-17 induction by Schistosoma japonicum infection in C57BL/6 mouse liver. Immunology. 2013; 139:523–532.8. Hammerich L, Tacke F. Role of gamma-delta T cells in liver inflammation and fibrosis. World J Gastrointest Pathophysiol. 2014; 5:107–113.9. Li WT, Zhang GH, Li JJ, Chang LH, Wang K, Yang QT. Gammadelta T cell expression and significance in chronic rhinosinusitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2013; 48:311–315.10. Hahn YS, Taube C, Jin N, Sharp L, Wands JM, Aydintug MK, et al. Different potentials of gamma delta T cell subsets in regulating airway responsiveness: V gamma 1+ cells, but not V gamma 4+ cells, promote airway hyperreactivity, Th2 cytokines, and airway inflammation. J Immunol. 2004; 172:2894–2902.11. Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012; 50:1–12.12. Cao PP, Li HB, Wang BF, Wang SB, You XJ, Cui YH, et al. Distinct immunopathologic characteristics of various types of chronic rhinosinusitis in adult Chinese. J Allergy Clin Immunol. 2009; 124:478–484. 484.e1–484.e2.13. Li Y, Chen S, Yang L, Li B, Chan JY, Cai D. TRGV and TRDV repertoire distribution and clonality of T cells from umbilical cord blood. Transpl Immunol. 2009; 20:155–162.14. Hulin A, Deroanne CF, Lambert CA, Dumont B, Castronovo V, Defraigne JO, et al. Metallothionein-dependent up-regulation of TGF-beta2 participates in the remodelling of the myxomatous mitral valve. Cardiovasc Res. 2012; 93:480–489.15. Hellquist HB. Nasal polyps update. Histopathology. Allergy Asthma Proc. 1996; 17:237–242.16. Zhang N, Van Zele T, Perez-Novo C, Van Bruaene N, Holtappels G, DeRuyck N, et al. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol. 2008; 122:961–968.17. Witherden DA, Havran WL. Molecular aspects of epithelial gammadelta T cell regulation. Trends Immunol. 2011; 32:265–271.18. Kabelitz D, Wesch D, Hinz T. Gamma delta T cells, their T cell receptor usage and role in human diseases. Springer Semin Immunopathol. 1999; 21:55–75.19. Sinkora M, Sinkorová J, Holtmeier W. Development of gammadelta thymocyte subsets during prenatal and postnatal ontogeny. Immunology. 2005; 115:544–555.20. Chodaczek G, Papanna V, Zal MA, Zal T. Body-barrier surveillance by epidermal gammadelta TCRs. Nat Immunol. 2012; 13:272–282.21. Jameson J, Havran WL. Skin gammadelta T-cell functions in homeostasis and wound healing. Immunol Rev. 2007; 215:114–122.22. Jameson JM, Cauvi G, Witherden DA, Havran WL. A keratinocyte-responsive gamma delta TCR is necessary for dendritic epidermal T cell activation by damaged keratinocytes and maintenance in the epidermis. J Immunol. 2004; 172:3573–3579.23. Cairo C, Arabito E, Landi F, Casati A, Brunetti E, Mancino G, et al. Analysis of circulating gammadelta T cells in children affected by IgE-associated and non-IgE-associated allergic atopic eczema/dermatitis syndrome. Clin Exp Immunol. 2005; 141:116–121.24. Krug N, Erpenbeck VJ, Balke K, Petschallies J, Tschernig T, Hohlfeld JM, et al. Cytokine profile of bronchoalveolar lavage-derived CD4(+), CD8(+), and gammadelta T cells in people with asthma after segmental allergen challenge. Am J Respir Cell Mol Biol. 2001; 25:125–131.25. Meltzer EO, Hamilos DL, Hadley JA, Lanza DC, Marple BF, Nicklas RA, et al. Rhinosinusitis: establishing definitions for clinical research and patient care. J Allergy Clin Immunol. 2004; 114:155–212.26. Kim JW, Hong SL, Kim YK, Lee CH, Min YG, Rhee CS. Histological and immunological features of non-eosinophilic nasal polyps. Otolaryngol Head Neck Surg. 2007; 137:925–930.27. Zhang N, Holtappels G, Claeys C, Huang G, van Cauwenberge P, Bachert C. Pattern of inflammation and impact of Staphylococcus aureus enterotoxins in nasal polyps from southern China. Am J Rhinol. 2006; 20:445–450.28. Bachert C, Zhang N, Holtappels G, De Lobel L, van Cauwenberge P, Liu S, et al. Presence of IL-5 protein and IgE antibodies to staphylococcal enterotoxins in nasal polyps is associated with comorbid asthma. J Allergy Clin Immunol. 2010; 126:962–968. 968.e1–968.e6.29. Huang Y, Jin N, Roark CL, Aydintug MK, Wands JM, Huang H, et al. The influence of IgE-enhancing and IgE-suppressive gammadelta T cells changes with exposure to inhaled ovalbumin. J Immunol. 2009; 183:849–855.30. Jinghong Z, Chaoqian L, Sujuan G, Yi L. Inhaled inactivated-Mycobacterium phlei modulates γδT cell function and alleviates airway inflammation in a mouse model of asthma. Asian Pac J Allergy Immunol. 2013; 31:286–291.31. Lo Re S, Dumoutier L, Couillin I, Van Vyve C, Yakoub Y, Uwambayinema F, et al. IL-17A-producing gammadelta T and Th17 lymphocytes mediate lung inflammation but not fibrosis in experimental silicosis. J Immunol. 2010; 184:6367–6377.32. Yokozeki H, Watanabe K, Igawa K, Miyazaki Y, Katayama I, Nishioka K. Gammadelta T cells assist alphabeta T cells in the adoptive transfer of contact hypersensitivity to para-phenylenediamine. Clin Exp Immunol. 2001; 125:351–359.33. Flood-Page P, Menzies-Gow A, Phipps S, Ying S, Wangoo A, Ludwig MS, et al. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Invest. 2003; 112:1029–1036.34. Figueiredo CR, Silva ID, Weckx LL. Inflammatory genes in nasal polyposis. Curr Opin Otolaryngol Head Neck Surg. 2008; 16:18–21.35. Li X, Meng J, Qiao X, Liu Y, Liu F, Zhang N, et al. Expression of TGF, matrix metalloproteinases, and tissue inhibitors in Chinese chronic rhinosinusitis. J Allergy Clin Immunol. 2010; 125:1061–1068.36. Li G, Zhang Y, Qian Y, Zhang H, Guo S, Sunagawa M, et al. Interleukin-17A promotes rheumatoid arthritis synoviocytes migration and invasion under hypoxia by increasing MMP2 and MMP9 expression through NF-kappaB/HIF-1alpha pathway. Mol Immunol. 2013; 53:227–236.37. Saitoh T, Kusunoki T, Yao T, Kawano K, Kojima Y, Miyahara K, et al. Role of interleukin-17A in the eosinophil accumulation and mucosal remodeling in chronic rhinosinusitis with nasal polyps associated with asthma. Int Arch Allergy Immunol. 2010; 151:8–16.38. Wakashin H, Hirose K, Maezawa Y, Kagami S, Suto A, Watanabe N, et al. IL-23 and Th17 cells enhance Th2-cell-mediated eosinophilic airway inflammation in mice. Am J Respir Crit Care Med. 2008; 178:1023–1032.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Significance of Serum Eosinophil Cationic Protein(ECP) in Chronic Rhinosinusitis with Nasal Polyposis
- Medical treatment according to phenotypes of chronic rhinosinusitis
- Comparison of Corticosteroids by 3 Approaches to the Treatment of Chronic Rhinosinusitis With Nasal Polyps
- Differences in MMP-2, MMP-9, TIMP-1, and eosinophil inflammatory markers of nasal polyp homogenates between aspirin intolerant and tolerant asthma
- Correlation between distribution of eosinophil cationic protein and nasal hyperreactivity in perennial allergic rhinitis