Endocrinol Metab.
2017 Mar;32(1):129-139. 10.3803/EnM.2017.32.1.129.
The Effects of Altered Membrane Cholesterol Levels on Sodium Pump Activity in Subclinical Hypothyroidism
- Affiliations
-
- 1Department of Biochemistry, Calcutta National Medical College, Kolkata, India. anindya653@gmail.com
- KMID: 2413297
- DOI: http://doi.org/10.3803/EnM.2017.32.1.129
Abstract
- BACKGROUND
Metabolic dysfunctions characteristic of overt hypothyroidism (OH) start at the early stage of subclinical hypothyroidism (SCH). Naâº/Kâº-ATPase (the sodium pump) is a transmembrane enzyme that plays a vital role in cellular activities in combination with membrane lipids. We evaluated the effects of early changes in thyroid hormone and membrane cholesterol on sodium pump activity in SCH and OH patients.
METHODS
In 32 SCH patients, 35 OH patients, and 34 euthyroid patients, sodium pump activity and cholesterol levels in red blood cell membranes were measured. Serum thyroxine (Tâ‚„) and thyroid stimulating hormone (TSH) levels were measured using enzyme-linked immunosorbent assays. Differences in their mean values were analysed using post hoc analysis of variance. We assessed the dependence of the sodium pump on other metabolites by multiple regression analysis.
RESULTS
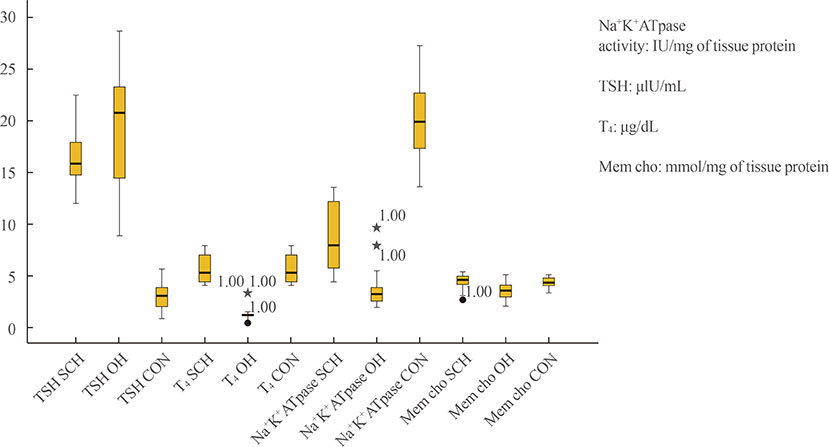
Sodium pump activity and membrane cholesterol were lower in both hypothyroid groups than in control group, OH group exhibiting lower values than SCH group. In SCH group, sodium pump activity showed a significant direct dependence on membrane cholesterol with an inverse relationship with serum TSH levels. In OH group, sodium pump activity depended directly on membrane cholesterol and serum T4 levels. No dependence on serum cholesterol was observed in either case.
CONCLUSION
Despite the presence of elevated serum cholesterol in hypothyroidism, membrane cholesterol contributed significantly to maintain sodium pump activity in the cells. A critical reduction in membrane cholesterol levels heralds compromised enzyme activity, even in the early stage of hypothyroidism, and this can be predicted by elevated TSH levels alone, without any evident clinical manifestations.
MeSH Terms
Figure
Reference
-
1. Efstathiadou Z, Bitsis S, Milionis HJ, Kukuvitis A, Bairaktari ET, Elisaf MS, et al. Lipid profile in subclinical hypothyroidism: is L-thyroxine substitution beneficial? Eur J Endocrinol. 2001; 145:705–710.2. Sharma R, Sharma TK, Kaushik GG, Sharma S, Vardey SK, Sinha M. Subclinical hypothyroidism and its association with cardiovascular risk factors. Clin Lab. 2011; 57:719–724.3. Biondi B. Mechanisms in endocrinology: heart failure and thyroid dysfunction. Eur J Endocrinol. 2012; 167:609–618.4. Wollenweber FA, Zietemann V, Gschwendtner A, Opherk C, Dichgans M. Subclinical hyperthyroidism is a risk factor for poor functional outcome after ischemic stroke. Stroke. 2013; 44:1446–1448.5. Roy S, Banerjee U, Dasgupta A. A comparative study to evaluate the interplay of lipoprotein (a) with traditional lipid parameters in overt and subclinical hypothyroidism. Br J Med Med Res. 2015; 10:1–11.6. Roy S, Banerjee U, Dasgupta A. Effect of sub clinical hypothyroidism on C-reactive protein and ischemia modified albumin. Mymensingh Med J. 2015; 24:379–384.7. Madathil A, Hollingsworth KG, Blamire AM, Razvi S, Newton JL, Taylor R, et al. Levothyroxine improves abnormal cardiac bioenergetics in subclinical hypothyroidism: a cardiac magnetic resonance spectroscopic study. J Clin Endocrinol Metab. 2015; 100:E607–E610.8. Muller MJ, Seitz HJ. Thyroid hormone action on intermediary metabolism. Part I: respiration, thermogenesis and carbohydrate metabolism. Klin Wochenschr. 1984; 62:11–18.9. Werneck FZ, Coelho EF, de Lima JR, Laterza MC, Barral MM, Teixeira Pde F, et al. Pulmonary oxygen uptake kinetics during exercise in subclinical hypothyroidism. Thyroid. 2014; 24:931–938.10. Liang M, Tian J, Liu L, Pierre S, Liu J, Shapiro J, et al. Identification of a pool of non-pumping Na/K-ATPase. J Biol Chem. 2007; 282:10585–10593.11. Liu L, Mohammadi K, Aynafshar B, Wang H, Li D, Liu J, et al. Role of caveolae in signal-transducing function of cardiac Na+/K+-ATPase. Am J Physiol Cell Physiol. 2003; 284:C1550–C1560.12. Liu L, Askari A. Beta-subunit of cardiac Na+-K+-ATPase dictates the concentration of the functional enzyme in caveolae. Am J Physiol Cell Physiol. 2006; 291:C569–C578.13. Tian J, Cai T, Yuan Z, Wang H, Liu L, Haas M, et al. Binding of Src to Na+/K+-ATPase forms a functional signaling complex. Mol Biol Cell. 2006; 17:317–326.14. Nakhoul F, Thompson CB, McDonough AA. Developmental change in Na,K-ATPase alpha1 and beta1 expression in normal and hypothyroid rat renal cortex. Am J Nephrol. 2000; 20:225–231.15. Kahaly GJ, Dillmann WH. Thyroid hormone action in the heart. Endocr Rev. 2005; 26:704–728.16. Kasturi S, Ismail-Beigi F. Effect of thyroid hormone on the distribution and activity of Na, K-ATPase in ventricular myocardium. Arch Biochem Biophys. 2008; 475:121–127.17. Carageorgiou H, Pantos C, Zarros A, Stolakis V, Mourouzis I, Cokkinos D, et al. Changes in acetylcholinesterase, Na+,K+-ATPase, and Mg2+-ATPase activities in the frontal cortex and the hippocampus of hyper- and hypothyroid adult rats. Metabolism. 2007; 56:1104–1110.18. Koromilas C, Liapi C, Zarros A, Tsela S, Zissis KM, Kalafatakis K, et al. Inhibition of Na(+),K(+)-ATPase in the hypothalamus, pons and cerebellum of the offspring rat due to experimentally-induced maternal hypothyroidism. J Matern Fetal Neonatal Med. 2015; 28:1438–1444.19. DeLuise M, Flier JS. Status of the red cell Na,K-pump in hyper- and hypothyroidism. Metabolism. 1983; 32:25–30.20. Nicolini G, Balzan S, Colzani R, Scarlattini M, Taddei MC, Iervasi G. Erythrocyte Na/K-ATPase is increased in subjects with subclinical hypothyroidism. Clin Endocrinol (Oxf). 2004; 60:705–710.21. Duntas LH. Thyroid disease and lipids. Thyroid. 2002; 12:287–293.22. Razvi S, Ingoe L, Keeka G, Oates C, McMillan C, Weaver JU. The beneficial effect of L-thyroxine on cardiovascular risk factors, endothelial function, and quality of life in subclinical hypothyroidism: randomized, crossover trial. J Clin Endocrinol Metab. 2007; 92:1715–1723.23. Ruggiero FM, Gnoni GV, Quagliariello E. Effect of hypothyroidism on the lipid composition of rat plasma and erythrocyte membranes. Lipids. 1987; 22:148–151.24. Brasitus TA, Dudeja PK. Effect of hypothyroidism on the lipid composition and fluidity of rat colonic apical plasma membranes. Biochim Biophys Acta. 1988; 939:189–196.25. Noori S, Zafar H, Mahboob T. Biochemical effectiveness of cocoa powder on electrolytes homeostasis, liver and cardiac specific enzymes and renal function. Pak J Nutr. 2009; 8:882–886.26. Macchia T, Mancinelli R, Barbini DA, Taggi F, Avico U, Cantafora A. Determination of membrane cholesterol in normal and pathological red blood cells. Clin Chim Acta. 1991; 199:59–67.27. Kumar AR, Kurup PA. Membrane Na+ K+ ATPase inhibition related dyslipidemia and insulin resistance in neuropsychiatric disorders. Indian J Physiol Pharmacol. 2001; 45:296–304.28. Ravi Kumar A, Kurup PA. Digoxin and membrane sodium potassium ATPase inhibition in cardiovascular disease. Indian Heart J. 2000; 52:315–318.29. Prasad R, Mond R, Jain S, Kaur G, Chugh KS. Modulation of ouabain sensitive sodium potassium pump of erythrocytes from patients with chronic renal failure: role of acute hemodialysis. Biochem Mol Biol Int. 1996; 40:1087–1094.30. Ismail-Beigi F, Edelman IS. The mechanism of the calorigenic action of thyroid hormone. Stimulation of Na plus + K plus-activated adenosinetriphosphatase activity. J Gen Physiol. 1971; 57:710–722.31. Bell RJ, Rivera-Woll L, Davison SL, Topliss DJ, Donath S, Davis SR. Well-being, health-related quality of life and cardiovascular disease risk profile in women with subclinical thyroid disease: a community-based study. Clin Endocrinol (Oxf). 2007; 66:548–556.32. Shao Y, Ojamaa K, Klein I, Ismail-Beigi F. Thyroid hormone stimulates Na, K-ATPase gene expression in the hemodynamically unloaded heterotopically transplanted rat heart. Thyroid. 2000; 10:753–759.33. Lambropoulos N, Garcia A, Clarke RJ. Stimulation of Na(+),K(+)-ATPase activity as a possible driving force in cholesterol evolution. J Membr Biol. 2016; 249:251–259.34. Namazi G, Pourfarzam M, Jamshidi Rad S, Movahedian Attar A, Sarrafzadegan N, Sadeghi M, et al. Association of the total cholesterol content of erythrocyte membranes with the severity of disease in stable coronary artery disease. Cholesterol. 2014; 2014:821686.35. Gottlieb MH. Rates of cholesterol exchange between human erythrocytes and plasma lipoproteins. Biochim Biophys Acta. 1980; 600:530–541.36. Fielding CJ, Fielding PE. Intracellular cholesterol transport. J Lipid Res. 1997; 38:1503–1521.37. Cornelius F, Habeck M, Kanai R, Toyoshima C, Karlish SJ. General and specific lipid-protein interactions in Na,K-ATPase. Biochim Biophys Acta. 2015; 1848:1729–1743.38. Thompson GR, Soutar AK, Spengel FA, Jadhav A, Gavigan SJ, Myant NB. Defects of receptor-mediated low density lipoprotein catabolism in homozygous familial hypercholesterolemia and hypothyroidism in vivo. Proc Natl Acad Sci U S A. 1981; 78:2591–2595.39. Caraccio N, Ferrannini E, Monzani F. Lipoprotein profile in subclinical hypothyroidism: response to levothyroxine replacement, a randomized placebo-controlled study. J Clin Endocrinol Metab. 2002; 87:1533–1538.40. Tian L, Song Y, Xing M, Zhang W, Ning G, Li X, et al. A novel role for thyroid-stimulating hormone: up-regulation of hepatic 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase expression through the cyclic adenosine monophosphate/protein kinase A/cyclic adenosine monophosphate-responsive element binding protein pathway. Hepatology. 2010; 52:1401–1409.41. Zhang T, Zhou L, Li CC, Shi H, Zhou X. TSH increases synthesis of hepatic ATP-binding cassette subfamily A member 1 in hypercholesterolemia. Biochem Biophys Res Commun. 2016; 476:75–81.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Profile of serum lipoprotein in patients with subclinical hypothyroidism
- Comparison of Common Carotid Artery Intima-Media Thickness between Subclinical Hypothyroidism and Euthyroidism
- Serum lipoprotein(a) and lipid concentrations in patients with subclinical hypothyroidism
- Subclinical Thyroid Dysfunction in the Elderly
- Plasma CRP, apolipoprotein A-1, apolipoprotein B and Lp(a) according to thyroid function status