J Gynecol Oncol.
2017 Nov;28(6):e77. 10.3802/jgo.2017.28.e77.
Prognostic value of programmed death-ligand 1 (PD-L1) expression in ovarian clear cell carcinoma
- Affiliations
-
- 1Department of Gynecologic Oncology, Fudan University Shanghai Cancer Center, Shanghai, China. docwuxh@yahoo.com
- 2Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China.
- 3Department of Pathology, Fudan University Shanghai Cancer Center, Shanghai, China.
- KMID: 2413203
- DOI: http://doi.org/10.3802/jgo.2017.28.e77
Abstract
OBJECTIVE
Programmed death-ligand 1 (PD-L1) was expressed in various tumors and antibodies targeting its receptor programmed cell death-1 (PD-1) are emerging cancer therapeutics. This study was designed to evaluate the expression of PD-L1 and its correlation with clinicopathologic features and clinical outcomes in ovarian clear cell carcinoma (OCCC).
METHODS
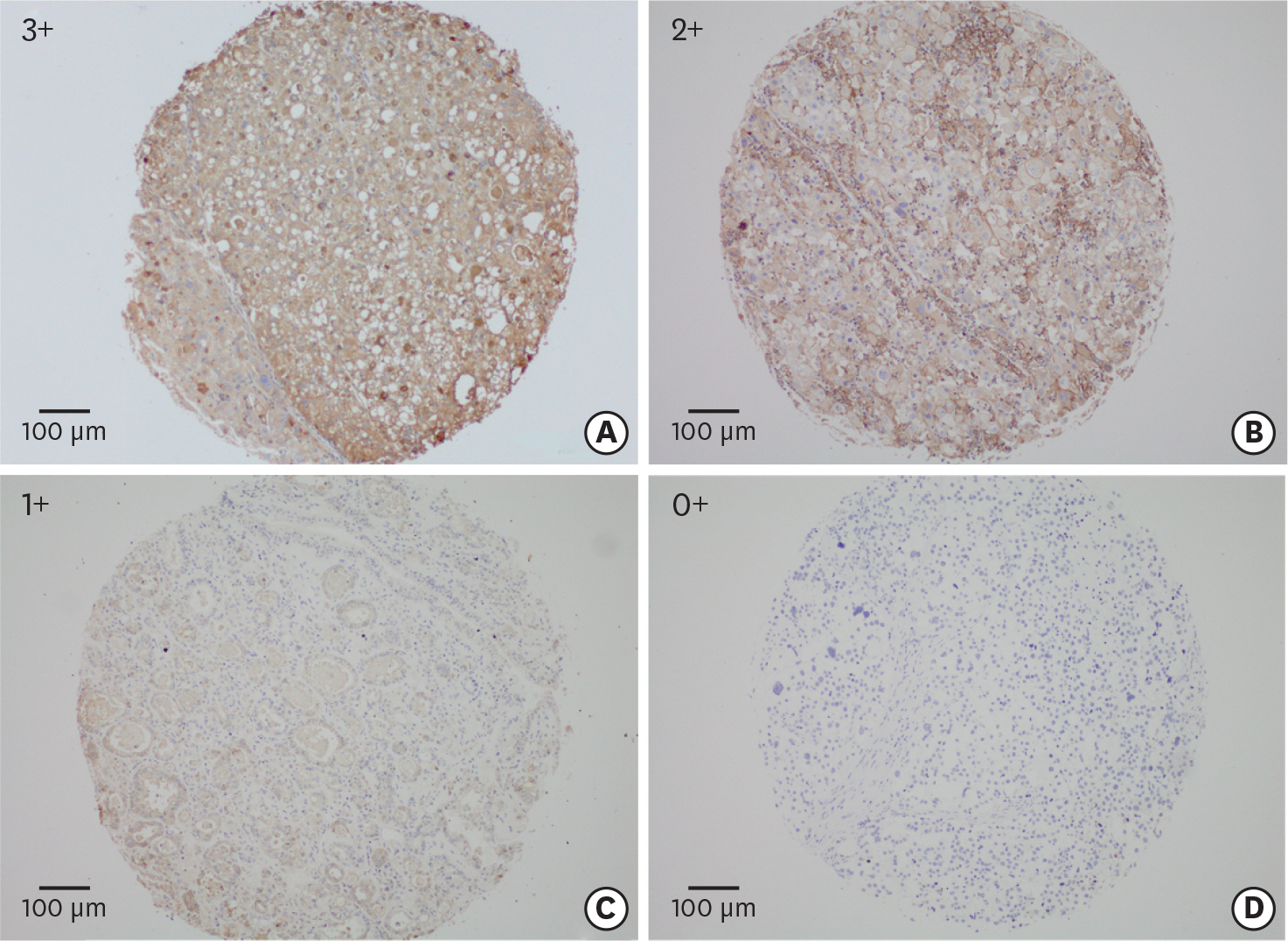
The PD-L1 expression was measured by tissue-microarray-based immunohistochemistry from 122 eligible patients diagnosed with OCCC. The associations of clinicopathologic features with progression-free survival (PFS) and overall survival (OS) were analyzed by Kaplan-Meier method and multivariate analysis was further performed by Cox regression model.
RESULTS
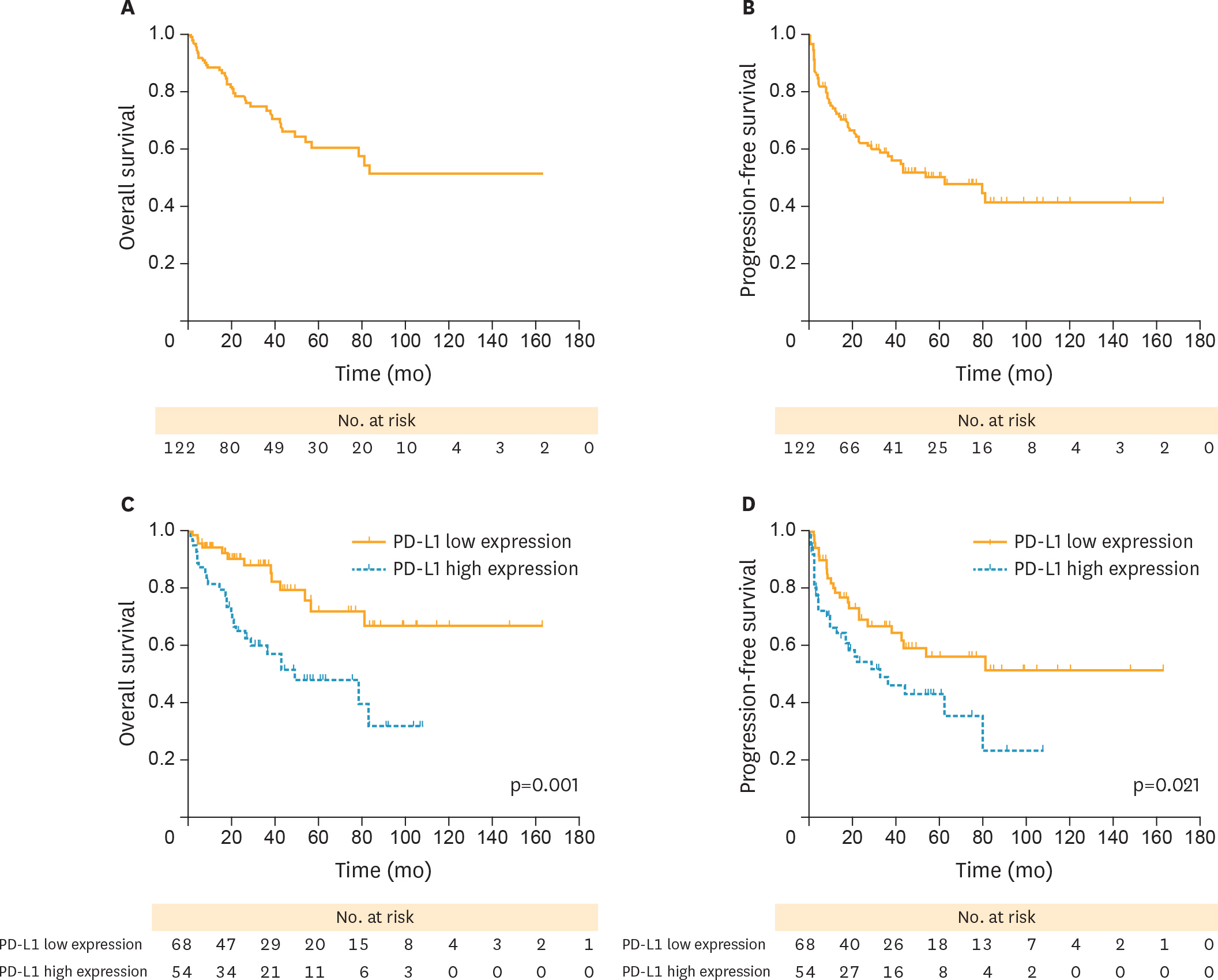
Overall, high PD-L1 expression (PD-L1(high)) was observed in 44.7% (55/123) of OCCC patients, and was strongly associated with advanced stages (p=0.020), positive ascitic fluid (p=0.016), platinum-resistant (PR) disease (p=0.045), and recurrence (p=0.038). Moreover, patients with PD-L1(high) were associated with poorer OS (hazard ratio [HR]=2.877; p=0.001) and PFS (HR=1.843; p=0.021) than those with low PD-L1 expression (PD-L1(low)). In subgroup analysis, PD-L1(high) patients experienced a poorer PFS (HR=1.926; p=0.044) and OS (HR=2.492; p=0.021) than PD-L1(low) cases among advanced stages (III-IV), but this difference was not observed in stage I-II patients. Meanwhile, PD-L1(high) was associated with poorer prognosis than PD-L1(low) in PR patients (OS, HR=2.253; p=0.037; PFS, HR=1.448; p=0.233). Multivariate analysis revealed that PD-L1(high) and advanced stages (III-IV) were adverse independent prognosticators for both PFS (HR(PD-L1)=2.0; p(PD-L1)=0.038; HR(stage)=10.2; p(stage)<0.001) and OS (HR(PD-L1)=3.0; p(PD-L1)=0.011; HR(stage)=14.3; p(stage)<0.001).
CONCLUSION
PD-L1(high) might serve as a risk factor for PFS and OS in patients with OCCC. It is possible that immunotherapy targeting PD-L1 pathway could be used in OCCC.
MeSH Terms
-
Adenocarcinoma, Clear Cell/*metabolism
Adult
Aged
Aged, 80 and over
B7-H1 Antigen/*metabolism
Disease-Free Survival
Female
Humans
Immunohistochemistry
Kaplan-Meier Estimate
Middle Aged
Multivariate Analysis
Ovarian Neoplasms/*metabolism
Prognosis
Proportional Hazards Models
Survival Rate
Tissue Array Analysis
Young Adult
B7-H1 Antigen
Figure
Cited by 1 articles
-
Prognostic value of programmed cell death ligand-1 expression in ovarian cancer: an updated meta-analysis
Jinlan Piao, Hyun Ji Lim, Maria Lee
Obstet Gynecol Sci. 2020;63(3):346-356. doi: 10.5468/ogs.2020.63.3.346.
Reference
-
References
1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012; 62:10–29.
Article2. Kurman RJ, Shih IM. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010; 34:433–43.
Article3. Takano M, Tsuda H, Sugiyama T. Clear cell carcinoma of the ovary: is there a role of histology-specific treatment? J Exp Clin Cancer Res. 2012; 31:53.
Article4. Chan JK, Teoh D, Hu JM, Shin JY, Osann K, Kapp DS. Do clear cell ovarian carcinomas have poorer prognosis compared to other epithelial cell types? A study of 1411 clear cell ovarian cancers. Gynecol Oncol. 2008; 109:370–6.
Article5. Ye S, Yang J, You Y, Cao D, Huang H, Wu M, et al. Comparison of clinical characteristic and prognosis between ovarian clear cell carcinoma and serous carcinoma: a 10-year cohort study of chinese patients. PLoS One. 2015; 10:e0133498.
Article6. Schnack TH, Høgdall E, Nedergaard L, Høgdall C. Demographic clinical and prognostic factors of primary ovarian adenocarcinomas of serous and clear cell histology-a comparative study. Int J Gynecol Cancer. 2016; 26:82–90.
Article7. Chen BJ, Chapuy B, Ouyang J, Sun HH, Roemer MG, Xu ML, et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res. 2013; 19:3462–73.
Article8. Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007; 19:813–24.
Article9. Philips GK, Atkins M. Therapeutic uses of anti-PD-1 and anti-PD-L1 antibodies. Int Immunol. 2015; 27:39–46.
Article10. Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000; 192:1027–34.
Article11. Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, et al. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A. 2004; 101:17174–9.
Article12. Liu J, Hamrouni A, Wolowiec D, Coiteux V, Kuliczkowski K, Hetuin D, et al. Plasma cells from multiple myeloma patients express B7-H1 (PD-L1) and increase expression after stimulation with IFN-{gamma} and TLR ligands via a MyD88-, TRAF6-, and MEK-dependent pathway. Blood. 2007; 110:296–304.13. Droeser RA, Hirt C, Viehl CT, Frey DM, Nebiker C, Huber X, et al. Clinical impact of programmed cell death ligand 1 expression in colorectal cancer. Eur J Cancer. 2013; 49:2233–42.
Article14. Lyford-Pike S, Peng S, Young GD, Taube JM, Westra WH, Akpeng B, et al. Evidence for a role of the PD-1: PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013; 73:1733–41.15. Chen YB, Mu CY, Huang JA. Clinical significance of programmed death-1 ligand-1 expression in patients with non-small cell lung cancer: a 5-year-follow-up study. Tumori. 2012; 98:751–5.
Article16. Shi SJ, Wang LJ, Wang GD, Guo ZY, Wei M, Meng YL, et al. B7-H1 expression is associated with poor prognosis in colorectal carcinoma and regulates the proliferation and invasion of HCT116 colorectal cancer cells. PLoS One. 2013; 8:e76012.
Article17. Muenst S, Schaerli AR, Gao F, Däster S, Trella E, Droeser RA, et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2014; 146:15–24.
Article18. Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007; 104:3360–5.
Article19. Darb-Esfahani S, Kunze CA, Kulbe H, Sehouli J, Wienert S, Lindner J, et al. Prognostic impact of programmed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor-infiltrating lymphocytes in ovarian high grade serous carcinoma. Oncotarget. 2016; 7:1486–99.
Article20. Webb JR, Milne K, Nelson BH. PD-1 and CD103 are widely coexpressed on prognostically favorable intraepithelial CD8 T cells in human ovarian cancer. Cancer Immunol Res. 2015; 3:926–35.
Article21. Böcker W. WHO classification of breast tumors and tumors of the female genital organs: pathology and genetics. Verh Dtsch Ges Pathol. 2002; 86:116–9.22. Nomi T, Sho M, Akahori T, Hamada K, Kubo A, Kanehiro H, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007; 13:2151–7.
Article23. Abiko K, Mandai M, Hamanishi J, Yoshioka Y, Matsumura N, Baba T, et al. PD-L1 on tumor cells is induced in ascites and promotes peritoneal dissemination of ovarian cancer through CTL dysfunction. Clin Cancer Res. 2013; 19:1363–74.
Article24. Maine CJ, Aziz NH, Chatterjee J, Hayford C, Brewig N, Whilding L, et al. Programmed death ligand-1 over-expression correlates with malignancy and contributes to immune regulation in ovarian cancer. Cancer Immunol Immunother. 2014; 63:215–24.
Article25. Tokito T, Azuma K, Kawahara A, Ishii H, Yamada K, Matsuo N, et al. Predictive relevance of PD-L1 expression combined with CD8+ TIL density in stage III non-small cell lung cancer patients receiving concurrent chemoradiotherapy. Eur J Cancer. 2016; 55:7–14.
Article26. Zhang Y, Kang S, Shen J, He J, Jiang L, Wang W, et al. Prognostic significance of programmed cell death 1 (PD-1) or PD-1 ligand 1 (PD-L1) Expression in epithelial-originated cancer: a meta-analysis. Medicine (Baltimore). 2015; 94:e515.27. Lussier DM, Johnson JL, Hingorani P, Blattman JN. Combination immunotherapy with α-CTLA-4 and α-PD-L1 antibody blockade prevents immune escape and leads to complete control of metastatic osteosarcoma. J Immunother Cancer. 2015; 3:21.
Article28. Tjin EP, Krebbers G, Meijlink KJ, van de Kasteele W, Rosenberg EH, Sanders J, et al. Immune-escape markers in relation to clinical outcome of advanced melanoma patients following immunotherapy. Cancer Immunol Res. 2014; 2:538–46.
Article29. Kluger HM, Zito CR, Barr ML, Baine MK, Chiang VL, Sznol M, et al. Characterization of PD-L1 expression and associated T-cell infiltrates in metastatic melanoma samples from variable anatomic sites. Clin Cancer Res. 2015; 21:3052–60.
Article30. Beckers RK, Selinger CI, Vilain R, Madore J, Wilmott JS, Harvey K, et al. Programmed death ligand 1 expression in triple-negative breast cancer is associated with tumour-infiltrating lymphocytes and improved outcome. Histopathology. 2016; 69:25–34.
Article31. Duraiswamy J, Freeman GJ, Coukos G. Therapeutic PD-1 pathway blockade augments with other modalities of immunotherapy T-cell function to prevent immune decline in ovarian cancer. Cancer Res. 2013; 73:6900–12.
Article32. Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012; 366:2455–65.
Article33. Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A, Murayama T, et al. Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2015; 33:4015–22.
Article34. Homicsko K, Coukos G. Targeting programmed cell death 1 in ovarian cancer. J Clin Oncol. 2015; 33:3987–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- PD-L1 Testing in Non-small Cell Lung Cancer: Past, Present, and Future
- Prognostic significance of programmed cell death-ligand 1 expression on immune cells and epithelialmesenchymal transition expression in patients with hepatocellular carcinoma
- Immunohistochemical expression of programmed death-ligand 1 and CD8 in glioblastomas
- Prognostic value of programmed cell death ligand-1 expression in ovarian cancer: an updated meta-analysis
- Human Leukocyte Antigen Class I and Programmed Death-Ligand 1 Coexpression Is an Independent Poor Prognostic Factor in Adenocarcinoma of the Lung