Ann Surg Treat Res.
2023 Nov;105(5):297-309. 10.4174/astr.2023.105.5.297.
Prognostic significance of programmed cell death-ligand 1 expression on immune cells and epithelialmesenchymal transition expression in patients with hepatocellular carcinoma
- Affiliations
-
- 1Department of Surgery, Soonchunhyang University Cheonan Hospital, Soonchunhyang University College of Medicine, Cheonan, Korea
- 2Department of Pathology, Soonchunhyang University Cheonan Hospital, Soonchunhyang University College of Medicine, Cheonan, Korea
- KMID: 2547535
- DOI: http://doi.org/10.4174/astr.2023.105.5.297
Abstract
- Purpose
Immune checkpoint inhibitors (ICIs) have been shown significant oncological improvements in several cancers. However, ICIs are still in their infancy in hepatocellular carcinoma (HCC). Programmed cell death-ligand 1 (PD-L1), tumorinfiltrating lymphocytes (TILs), and epithelial-mesenchymal transition (EMT) have been known as prognostic factors in HCC. Therefore, we have focused on identifying the molecular mechanisms between each marker to evaluate a predictive role.
Methods
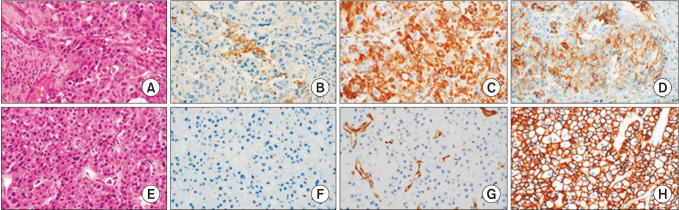
Formalin-fixed paraffin-embedded samples were obtained from 166 patients with HCC who underwent surgery. The expression of PD-L1 and TILs and EMT marker were evaluated by immunohistochemical analysis.
Results
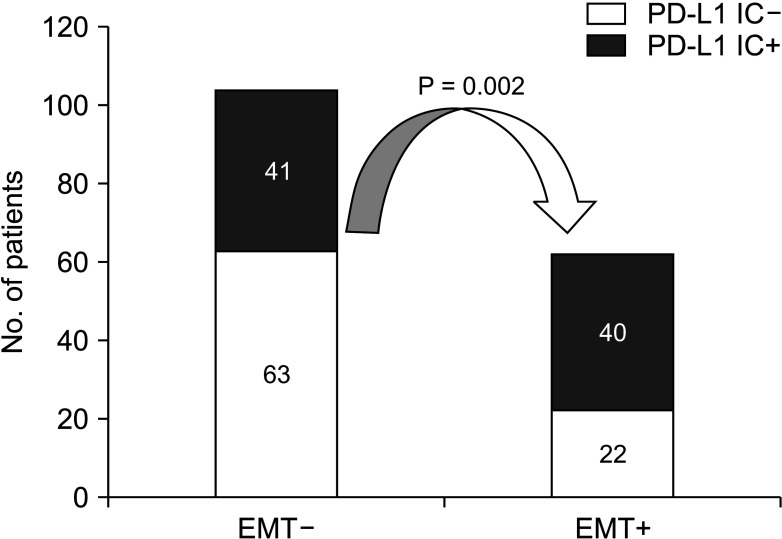
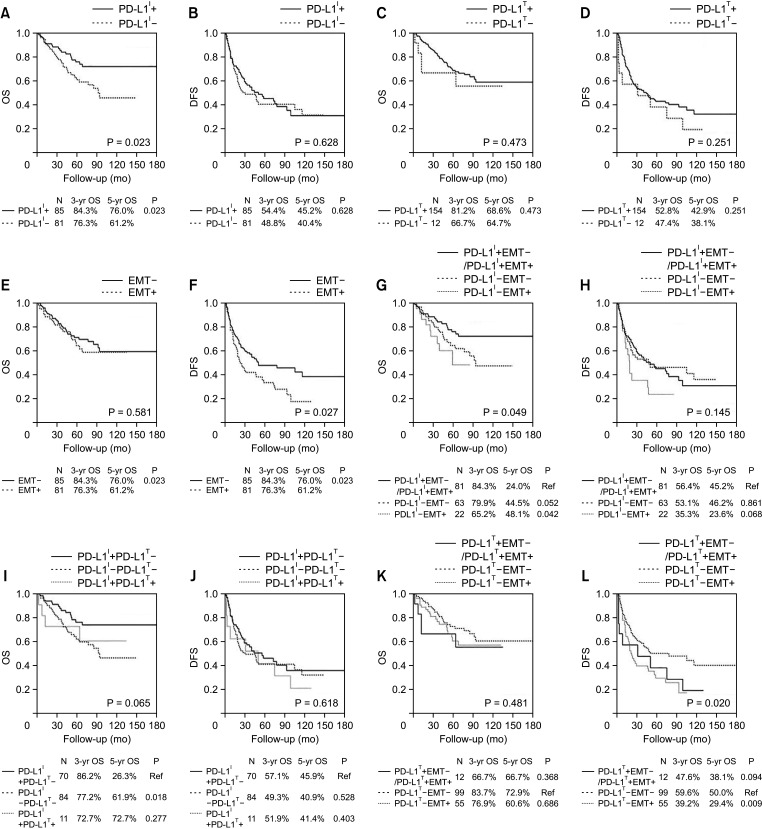
The multivariate analysis showed that TIL expression (hazard ratio [HR], 0.483; 95% confidence interval [CI], 0.269–0.866; P = 0.015) were independent prognostic factors for overall survival. The prognostic factors for disease-free survival were EMT marker expression (HR, 1.565; 95% CI, 1.019–2.403; P = 0.005). Patients with high expression of TILs had significantly better survival compared to patients with low expression (P = 0.023). Patients who were TIL+/EMT– showed a significantly better prognosis than those who were TIL–/EMT+ (P = 0.049).
Conclusion
This study demonstrates that PD-L1 expression of TILs is closely associated with EMT marker expression in HCC. Clinical investigations using anti–PD-1/PD-L1 inhibitors in patients with EMT-associated PD-L1 upregulation are warranted.
Keyword
Figure
Reference
-
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019; 69:7–34. PMID: 30620402.
Article2. Xu W, Liu K, Chen M, Sun JY, McCaughan GW, Lu XJ, et al. Immunotherapy for hepatocellular carcinoma: recent advances and future perspectives. Ther Adv Med Oncol. 2019; 11:1758835919862692. PMID: 31384311.
Article3. Jiang Y, Zhan H. Communication between EMT and PD-L1 signaling: new insights into tumor immune evasion. Cancer Lett. 2020; 468:72–81. PMID: 31605776.
Article4. Zhang SC, Hu ZQ, Long JH, Zhu GM, Wang Y, Jia Y, et al. Clinical implications of tumor-infiltrating immune cells in breast cancer. J Cancer. 2019; 10:6175–6184. PMID: 31762828.
Article5. Clark WH Jr, Elder DE, Guerry D 4th, Braitman LE, Trock BJ, Schultz D, et al. Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst. 1989; 81:1893–1904. PMID: 2593166.
Article6. Xu X, Tan Y, Qian Y, Xue W, Wang Y, Du J, et al. Clinicopathologic and prognostic significance of tumor-infiltrating CD8+ T cells in patients with hepatocellular carcinoma: a meta-analysis. Medicine (Baltimore). 2019; 98:e13923. PMID: 30633166.7. Nam SJ, Kim YH, Park JE, Ra YS, Khang SK, Cho YH, et al. Tumor-infiltrating immune cell subpopulations and programmed death ligand 1 (PD-L1) expression associated with clinicopathological and prognostic parameters in ependymoma. Cancer Immunol Immunother. 2019; 68:305–318. PMID: 30483834.
Article8. Du B, Shim JS. Targeting epithelial-mesenchymal transition (EMT) to overcome drug resistance in cancer. Molecules. 2016; 21:965. PMID: 27455225.
Article9. Manjunath Y, Upparahalli SV, Avella DM, Deroche CB, Kimchi ET, Staveley-O’Carroll KF, et al. PD-L1 expression with epithelial mesenchymal transition of circulating tumor cells is associated with poor survival in curatively resected non-small cell lung cancer. Cancers (Basel). 2019; 11:806. PMID: 31212653.
Article10. Chen L, Xiong Y, Li J, Zheng X, Zhou Q, Turner A, et al. PD-L1 expression promotes epithelial to mesenchymal transition in human esophageal cancer. Cell Physiol Biochem. 2017; 42:2267–2280. PMID: 28848143.
Article11. Ock CY, Kim S, Keam B, Kim M, Kim TM, Kim JH, et al. PD-L1 expression is associated with epithelial-mesenchymal transition in head and neck squamous cell carcinoma. Oncotarget. 2016; 7:15901–15914. PMID: 26893364.
Article12. Kim S, Koh J, Kim MY, Kwon D, Go H, Kim YA, et al. PD-L1 expression is associated with epithelial-to-mesenchymal transition in adenocarcinoma of the lung. Hum Pathol. 2016; 58:7–14. PMID: 27473266.
Article13. Dai X, Xue J, Hu J, Yang SL, Chen GG, Lai PB, et al. Positive expression of programmed death ligand 1 in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. Transl Oncol. 2017; 10:511–517. PMID: 28558264.
Article14. Li JH, Ma WJ, Wang GG, Jiang X, Chen X, Wu L, et al. clinicopathologic significance and prognostic value of programmed cell death ligand 1 (PD-L1) in patients with hepatocellular carcinoma: a meta-analysis. Front Immunol. 2018; 9:2077. PMID: 30254644.
Article15. Fu J, Zhang Z, Zhou L, Qi Z, Xing S, Lv J, et al. Impairment of CD4+ cytotoxic T cells predicts poor survival and high recurrence rates in patients with hepatocellular carcinoma. Hepatology. 2013; 58:139–149. PMID: 22961630.
Article16. Chew V, Chen J, Lee D, Loh E, Lee J, Lim KH, et al. Chemokine-driven lymphocyte infiltration: an early intratumoural event determining long-term survival in resectable hepatocellular carcinoma. Gut. 2012; 61:427–438. PMID: 21930732.
Article17. Ding W, Xu X, Qian Y, Xue W, Wang Y, Du J, et al. Prognostic value of tumor-infiltrating lymphocytes in hepatocellular carcinoma: a meta-analysis. Medicine (Baltimore). 2018; 97:e13301. PMID: 30557978.18. Ogunwobi OO, Harricharran T, Huaman J, Galuza A, Odumuwagun O, Tan Y, et al. Mechanisms of hepatocellular carcinoma progression. World J Gastroenterol. 2019; 25:2279–2293. PMID: 31148900.
Article19. Gurzu S, Kobori L, Fodor D, Jung I. Epithelial mesenchyma l and endothelial mesenchymal transitions in hepatocellular carcinoma: a review. Biomed Res Int. 2019; 2019:2962580. PMID: 31781608.20. Jiang Y, Lo AW, Wong A, Chen W, Wang Y, Lin L, et al. Prognostic significance of tumor-infiltrating immune cells and PD-L1 expression in esophageal squamous cell carcinoma. Oncotarget. 2017; 8:30175–30189. PMID: 28404915.
Article21. Teng MW, Ngiow SF, Ribas A, Smyth MJ. Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res. 2015; 75:2139–2145. PMID: 25977340.
Article22. Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013; 369:122–133. PMID: 23724867.
Article23. Yan L, Xu F, Dai CL. Relationship between epithelial-to-mesenchymal transition and the inflammatory microenvironment of hepatocellular carcinoma. J Exp Clin Cancer Res. 2018; 37:203. PMID: 30157906.
Article24. Bouillez A, Rajabi H, Jin C, Samur M, Tagde A, Alam M, et al. MUC1-C integrates PD-L1 induction with repression of immune effectors in non-small-cell lung cancer. Oncogene. 2017; 36:4037–4046. PMID: 28288138.
Article25. Alsuliman A, Colak D, Al-Harazi O, Fitwi H, Tulbah A, Al-Tweigeri T, et al. Bidirectional crosstalk between PD-L1 expression and epithelial to mesenchymal transition: significance in claudin-low breast cancer cells. Mol Cancer. 2015; 14:149. PMID: 26245467.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Expression of Programmed Death-Ligand 1 on Immune Cells Is Related to a Better Prognosis in Biliary Tract Cancer
- Exploring the mythical abscopal effect: Radiation and programmed cell death protein 1 (PD-1) blockade for hepatocellular carcinoma
- Human Leukocyte Antigen Class I and Programmed Death-Ligand 1 Coexpression Is an Independent Poor Prognostic Factor in Adenocarcinoma of the Lung
- C4orf46 is a Potential Prognostic Biomarker for Liver Hepatocellular Carcinoma
- Overexpression of PD-L1 and PD-L2 Is Associated with Poor Prognosis in Patients with Hepatocellular Carcinoma