J Vet Sci.
2016 Sep;17(3):385-390. 10.4142/jvs.2016.17.3.385.
Establishment of a canine mammary gland tumor cell line and characterization of its miRNA expression
- Affiliations
-
- 1Joint Department of Veterinary Clinical Medicine, School of Veterinary Medicine, Faculty of Agriculture, Tottori University, Tottori 680-8553, Japan. tosaki@muses.tottori-u.ac.jp
- KMID: 2413139
- DOI: http://doi.org/10.4142/jvs.2016.17.3.385
Abstract
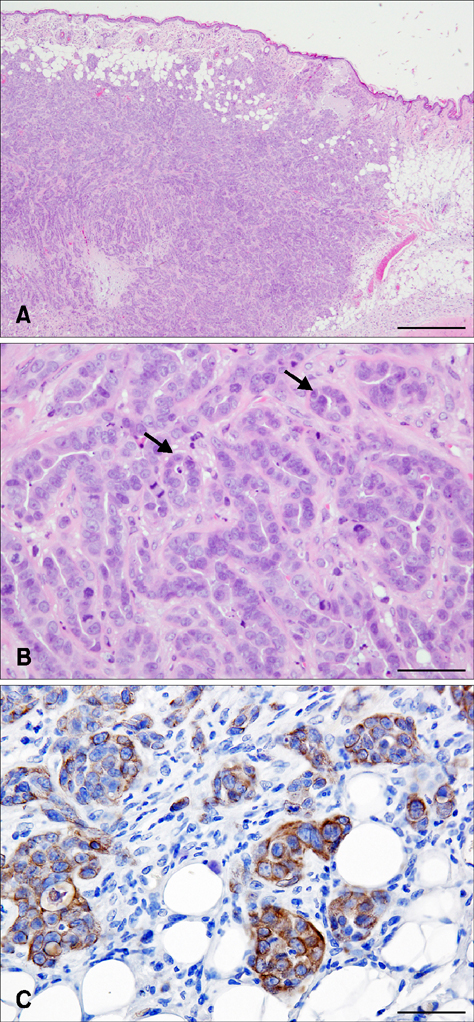
- Canine mammary gland tumors (CMGTs), which are the most common neoplasms in sexually intact female dogs, have been suggested as a model for studying human breast cancer because of several similarities, including relative age of onset, risk factors, incidence, histological and molecular features, biological behavior, metastatic pattern, and responses to therapy. In the present study, we established a new cell line, the SNP cell line, from a CMGT. A tumor formed in each NOD.CB17-Prkdc (scid)/J mouse at the site of subcutaneous SNP cell injection. SNP cells are characterized by proliferation in a tubulopapillary pattern and are vimentin positive. Moreover, we examined miRNA expression in the cultured cells and found that the expression values of miRNA-143 and miRNA-138a showed the greatest increase and decrease, respectively, of all miRNAs observed, indicating that these miRNAs might play a significant role in the malignancy of SNP cells. Overall, the results of this study indicate that SNP cells might serve as a model for future genetic analysis and clinical treatments of human breast tumors.
Keyword
MeSH Terms
Figure
Reference
-
1. Ambros V, Bartel B, Bartel DP, Burge CB, Carrington JC, Chen X, Dreyfuss G, Eddy SR, Griffiths-Jones S, Marshall M, Matzke M, Ruvkun G, Tuschl T. A uniform system for microRNA annotation. RNA. 2003; 9:277–279.
Article2. Blenkiron C, Goldstein LD, Thorne NP, Spiteri I, Chin SF, Dunning MJ, Barbosa-Morais NL, Teschendorff AE, Green AR, Ellis IO, Tavaré S, Caldas C, Miska EA. MicroRNA expression profiling of human breast cancer identifies new markers of tumor subtype. Genome Biol. 2007; 8:R214.
Article3. Boggs RM, Wright ZM, Stickney MJ, Porter WW, Murphy KE. MicroRNA expression in canine mammary cancer. Mamm Genome. 2008; 19:561–569.
Article4. Iorio MV, Ferracin M, Liu CG, Veronese A, Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M, Ménard S, Palazzo JP, Rosenberg A, Musiani P, Volinia S, Nenci I, Calin GA, Querzoli P, Negrini M, Croce CM. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005; 65:7065–7070.
Article5. Jiang J, Lee EJ, Gusev Y, Schmittgen TD. Real-time expression profiling of microRNA precursors in human cancer cell lines. Nucleic Acids Res. 2005; 33:5394–5403.
Article6. Lee YC, Tzeng WF, Chiou TJ, Chu ST. MicroRNA-138 suppresses neutrophil gelatinase-associated lipocalin expression and inhibits tumorigenicity. PLoS One. 2012; 7:e52979.
Article7. Liu X, Jiang L, Wang A, Yu J, Shi F, Zhou X. MicroRNA-138 suppresses invasion and promotes apoptosis in head and neck squamous cell carcinoma cell lines. Cancer Lett. 2009; 286:217–222.
Article8. Liu X, Wang C, Chen Z, Jin Y, Wang Y, Kolokythas A, Dai Y, Zhou X. MicroRNA-138 suppresses epithelial-mesenchymal transition in squamous cell carcinoma cell lines. Biochem J. 2011; 440:23–31.
Article9. Łosiewicz K, Chmielewska-Krzesińska M, Socha P, Jakimiuk A, Wąsowicz K. MiRNA-21, miRNA-10b, and miRNA-34a expression in canine mammary gland neoplasms. Bull Vet Inst Pulawy. 2014; 58:447–451.
Article10. Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007; 449:682–688.
Article11. Matos AJ, Baptista CS, Gärtner MF, Rutteman GR. Prognostic studies of canine and feline mammary tumours: the need for standardized procedures. Vet J. 2012; 193:24–31.
Article12. Mattie MD, Benz CC, Bowers J, Sensinger K, Wong L, Scott GK, Fedele V, Ginzinger D, Getts R, Haqq C. Optimized high-throughput microRNA expression profiling provides novel biomarker assessment of clinical prostate and breast cancer biopsies. Mol Cancer. 2006; 5:24.13. Miller TE, Ghoshal K, Ramaswamy B, Roy S, Datta J, Shapiro CL, Jacob S, Majumder S. MicroRNA-221/222 confers tamoxifen resistance in breast cancer by targeting p27Kip1. J Biol Chem. 2008; 283:29897–29903.
Article14. Mitomo S, Maesawa C, Ogasawara S, Iwaya T, Shibazaki M, Yashima-Abo A, Kotani K, Oikawa H, Sakurai E, Izutsu N, Kato K, Komatsu H, Ikeda K, Wakabayashi G, Masuda T. Downregulation of miR-138 is associated with overexpression of human telomerase reverse transcriptase protein in human anaplastic thyroid carcinoma cell lines. Cancer Sci. 2008; 99:280–286.
Article15. Noguchi S, Mori T, Hoshino Y, Yamada N, Nakagawa T, Sasaki N, Akao Y, Maruo K. Comparative study of anti-oncogenic microRNA-145 in canine and human malignant melanoma. J Vet Med Sci. 2012; 74:1–8.
Article16. Shafiee R, Javanbakht J, Atyabi N, Kheradmand P, Kheradmand D, Bahrami A, Daraei H, Khadivar F. Diagnosis, classification and grading of canine mammary tumours as a model to study human breast cancer: an clinico-cytohistopathological study with environmental factors influencing public health and medicine. Cancer Cell Int. 2013; 13:79.17. Shenouda SK, Alahari SK. MicroRNA function in cancer: oncogene or a tumor suppressor? Cancer Metastasis Rev. 2009; 28:369–378.
Article18. Sorenmo KU, Worley DR, Goldschmidt MH. Tumors of the mammary gland. In : Withrow SJ, Vail DM, Page RL, editors. Withrow and MacEwen's Small Animal Clinical Oncology. 5th ed. St Louis: Elsevier;2013. p. 538–556.19. Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, Prueitt RL, Yanaihara N, Lanza G, Scarpa A, Vecchione A, Negrini M, Harris CC, Croce CM. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006; 103:2257–2261.
Article20. von Deetzen MC, Schmeck BT, Gruber AD, Klopfleisch R. Malignancy associated microRNA expression changes in canine mammary cancer of different malignancies. ISRN Vet Sci. 2014; 2014:148597.
Article21. Voutsadakis IA. The network of pluripotency, epithelial-mesenchymal transition, and prognosis of breast cancer. Breast Cancer (Dove Med Press). 2015; 7:303–319.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Differential and correlated expressions of p16/p21/p27/p38 in mammary gland tumors of aged dogs
- Stage-specific embryonic antigen: determining expression in canine glioblastoma, melanoma, and mammary cancer cells
- Assessment of prognostic factors in dogs with mammary gland tumors: 60 cases (2014-2020)
- In vitro evaluation of the antitumor activity of axitinib in canine mammary gland tumor cell lines
- Plasma free amino acid profiles of canine mammary gland tumors