Clin Exp Otorhinolaryngol.
2018 Jun;11(2):133-140. 10.21053/ceo.2017.01389.
Impact and Modulations of Peripheral and Edaphic B Cell Subpopulations in Chronic Rhinosinusitis With Nasal Polyposis
- Affiliations
-
- 1Department of Oto-Rhino-Laryngology, Plastic, Aesthetic and Reconstructive Head and Neck Surgery, University of Wuerzburg, Wuerzburg, Germany. ickrath_p@ukw.de
- 2Institute for Virology and Immunobiology, University of Wuerzburg, Wuerzburg, Germany.
- 3Department of Oto-Rhino-Laryngology, Head and Neck Surgery “Otto Körnerâ€, University Medical Center Rostock, Rostock, Germany.
- KMID: 2412671
- DOI: http://doi.org/10.21053/ceo.2017.01389
Abstract
OBJECTIVES
The pathophysiological mechanisms of chronic rhinosinusitis with nasal polyposis (CRSwNP) still are discussed controversially. Regulatory B cells (Breg) are responsible for the suppression of T cell activity: deficiencies for Breg have been demonstrated to contribute to autoimmune disorders, e.g., systemic lupus erythematosus. In order to evaluate the influence of B cell subpopulations, especially Breg, on the etiology of this disease, the aim of this study was to characterize subpopulations of peripheral and edaphic B cells in CRSwNP.
METHODS
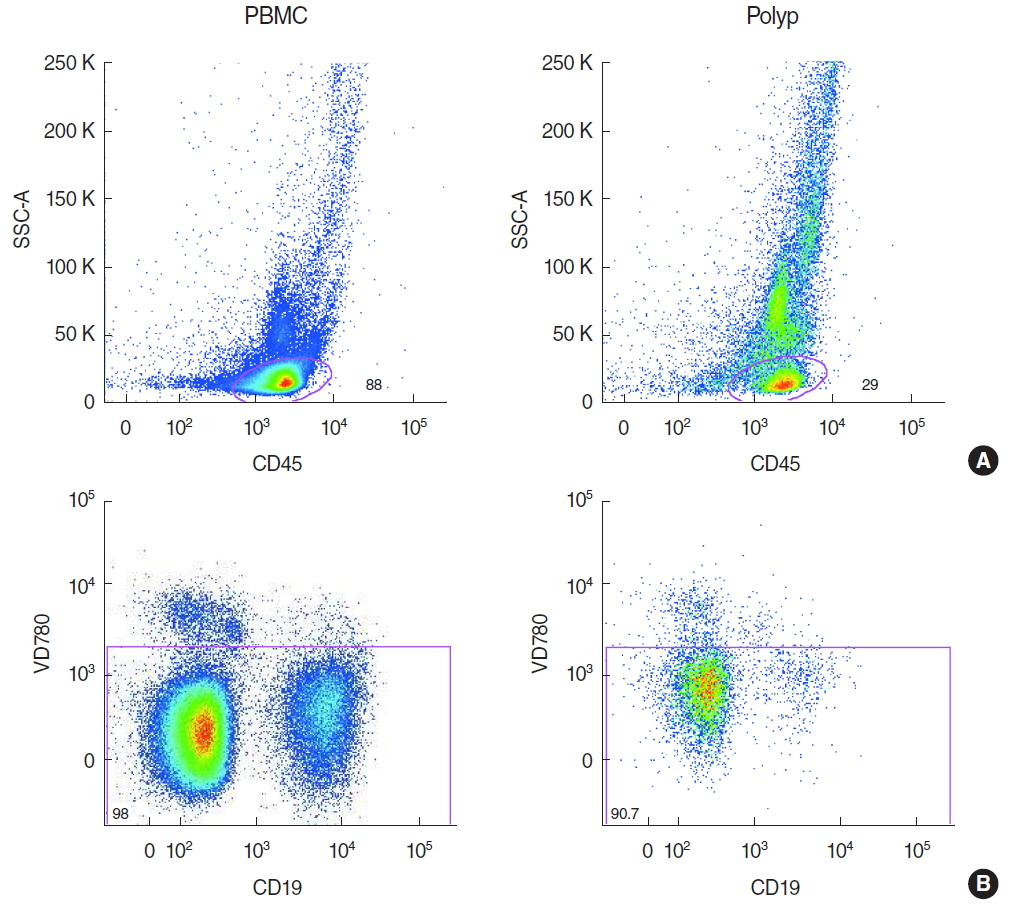
Polypoid tissue and blood samples were collected from 10 patients undergoing paranasal sinus surgery and lymphocytes were analyzed by multicolor flow cytometry.
RESULTS
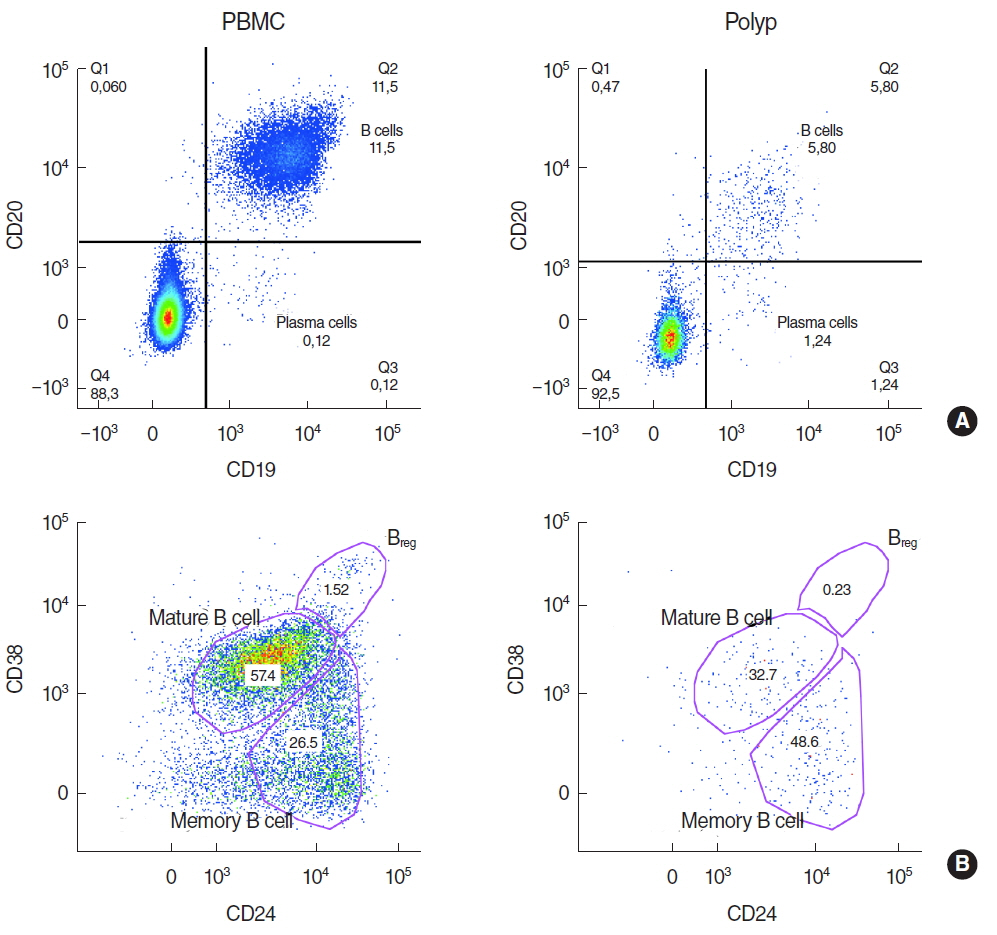
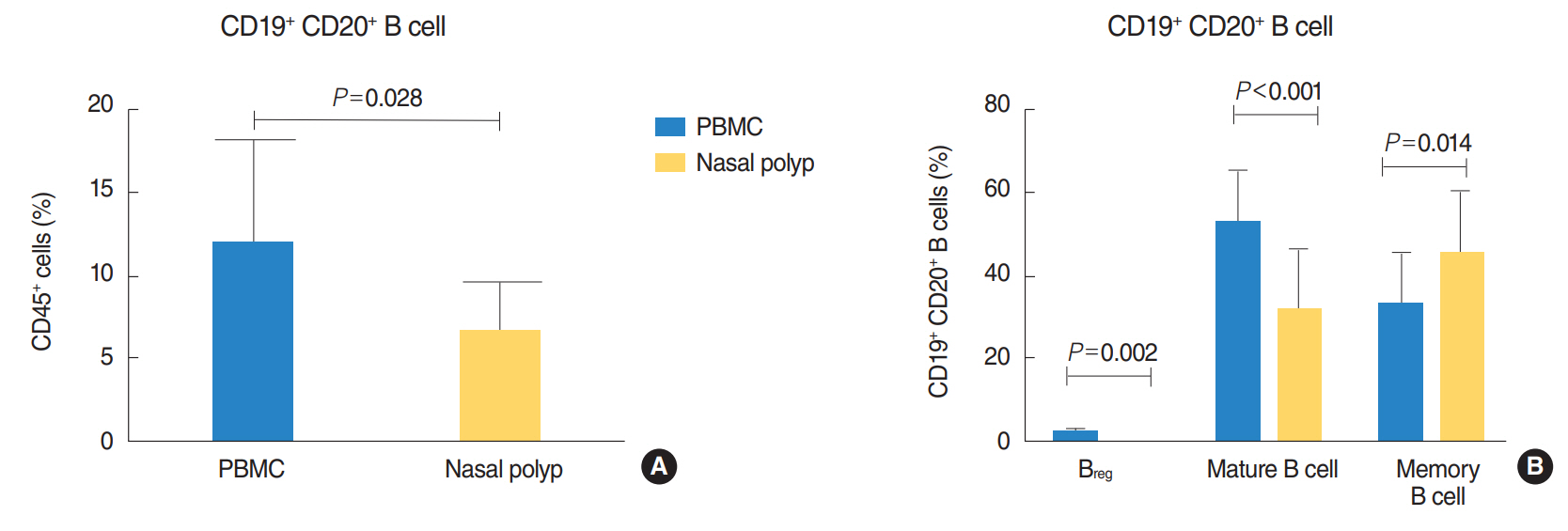
There was a significantly lower frequency of B cells in nasal polyps compared to peripheral blood mononuclear cells (PBMC) in patients with CRSwNP. Mature resting B cells were the main population within B cells in PBMC, and memory B cells in nasal polyps. Remarkably, Breg and mature B cells significantly decreased in nasal polyps compared to PBMC. Memory B cells significantly increased and represented the main subpopulation in nasal polyps in patients with CRSwNP.
CONCLUSION
In this study a detailed contemporary characterization of B cell subpopulations in patients with CRSwNP is presented. The influence of edaphic B cells could play a key role in the maintenance of this chronic infectious disease.
MeSH Terms
Figure
Reference
-
1. Larsen PL, Tos M. Origin of nasal polyps. Laryngoscope. 1991; Mar. 101(3):305–12.
Article2. Andrews AE, Bryson JM, Rowe-Jones JM. Site of origin of nasal polyps: relevance to pathogenesis and management. Rhinology. 2005; Sep. 43(3):180–4.3. Bachert C, Zhang L, Gevaert P. Current and future treatment options for adult chronic rhinosinusitis: focus on nasal polyposis. J Allergy Clin Immunol. 2015; Dec. 136(6):1431–40.
Article4. Ickrath P, Kleinsasser N, Ding X, Ginzkey C, Beyersdorf N, Hagen R, et al. Characterization of T-cell subpopulations in patients with chronic rhinosinusitis with nasal polyposis. Allergy Rhinol (Providence). 2017; Oct. 8(3):139–47.5. Dutsch-Wicherek M, Tomaszewska R, Strek P, Wicherek L, Skladzien J. The analysis of RCAS1 and DFF-45 expression in nasal polyps with respect to immune cells infiltration. BMC Immunol. 2006; Mar. 7:4.6. Zhang LP, Lin L, Zheng CQ, Shi GY. T-lymphocyte subpopulations and B7-H1/PD-1 expression in nasal polyposis. J Int Med Res. 2010; Mar-Apr. 38(2):593–601.
Article7. Dutsch-Wicherek M, Tomaszewska R, Lazar A, Strek P, Wicherek L, Kijowski J, et al. The presence of B7-H4+ macrophages and CD25+CD4+ and FOXP3+ regulatory T cells in the microenvironment of nasal polyps: a preliminary report. Folia Histochem Cytobiol. 2010; Dec. 48(4):611–7.
Article8. Gevaert P, Nouri-Aria KT, Wu H, Harper CE, Takhar P, Fear DJ, et al. Local receptor revision and class switching to IgE in chronic rhinosinusitis with nasal polyps. Allergy. 2013; Jan. 68(1):55–63.
Article9. Van Zele T, Gevaert P, Holtappels G, van Cauwenberge P, Bachert C. Local immunoglobulin production in nasal polyposis is modulated by superantigens. Clin Exp Allergy. 2007; Dec. 37(12):1840–7.
Article10. Bachert C, Zhang N, Holtappels G, De Lobel L, van Cauwenberge P, Liu S, et al. Presence of IL-5 protein and IgE antibodies to staphylococcal enterotoxins in nasal polyps is associated with comorbid asthma. J Allergy Clin Immunol. 2010; Nov. 126(5):962–8.e1-6.
Article11. Hoyer BF, Manz RA, Radbruch A, Hiepe F. Long-lived plasma cells and their contribution to autoimmunity. Ann N Y Acad Sci. 2005; Jun. 1050:124–33.
Article12. Blair PA, Norena LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity. 2010; Jan. 32(1):129–40.
Article13. Odendahl M, Mei H, Hoyer BF, Jacobi AM, Hansen A, Muehlinghaus G, et al. Generation of migratory antigen-specific plasma blasts and mobilization of resident plasma cells in a secondary immune response. Blood. 2005; Feb. 105(4):1614–21.
Article14. Jacobi AM, Odendahl M, Reiter K, Bruns A, Burmester GR, Radbruch A, et al. Correlation between circulating CD27high plasma cells and disease activity in patients with systemic lupus erythematosus. Arthritis Rheum. 2003; May. 48(5):1332–42.15. Robinson DS. The role of regulatory T lymphocytes in asthma pathogenesis. Curr Allergy Asthma Rep. 2005; Mar. 5(2):136–41.
Article16. Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009; Jun. 30(6):899–911.17. Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012; Mar. 50(1):1–12.
Article18. Derycke L, Eyerich S, Van Crombruggen K, Perez-Novo C, Holtappels G, Deruyck N, et al. Mixed T helper cell signatures in chronic rhinosinusitis with and without polyps. PLoS One. 2014; Jun. 9(6):e97581.
Article19. Kato A, Truong-Tran AQ, Scott AL, Matsumoto K, Schleimer RP. Airway epithelial cells produce B cell-activating factor of TNF family by an IFN-beta-dependent mechanism. J Immunol. 2006; Nov. 177(10):7164–72.20. Kato A, Peters A, Suh L, Carter R, Harris KE, Chandra R, et al. Evidence of a role for B cell-activating factor of the TNF family in the pathogenesis of chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2008; Jun. 121(6):1385–92.e1-2.
Article21. Patadia M, Dixon J, Conley D, Chandra R, Peters A, Suh LA, et al. Evaluation of the presence of B-cell attractant chemokines in chronic rhinosinusitis. Am J Rhinol Allergy. 2010; Jan-Feb. 24(1):11–6.
Article22. Peters AT, Kato A, Zhang N, Conley DB, Suh L, Tancowny B, et al. Evidence for altered activity of the IL-6 pathway in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2010; Feb. 125(2):397–403.e10.
Article23. Weksler ME. Changes in the B-cell repertoire with age. Vaccine. 2000; Feb. 18(16):1624–8.
Article24. Agematsu K, Nagumo H, Yang FC, Nakazawa T, Fukushima K, Ito S, et al. B cell subpopulations separated by CD27 and crucial collaboration of CD27+ B cells and helper T cells in immunoglobulin production. Eur J Immunol. 1997; Aug. 27(8):2073–9.
Article25. Belkaid Y, Tarbell K. Regulatory T cells in the control of host-microorganism interactions (*). Annu Rev Immunol. 2009; Apr. 27:551–89.26. Tan BK, Li QZ, Suh L, Kato A, Conley DB, Chandra RK, et al. Evidence for intranasal antinuclear autoantibodies in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2011; Dec. 128(6):1198–206.e1.
Article27. Gevaert P, Calus L, Van Zele T, Blomme K, De Ruyck N, Bauters W, et al. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. J Allergy Clin Immunol. 2013; Jan. 131(1):110–6.e1.
Article28. Dorner T, Radbruch A, Burmester GR. B-cell-directed therapies for autoimmune disease. Nat Rev Rheumatol. 2009; Aug. 5(8):433–41.
Article29. Murray T, Fuertes Marraco SA, Baumgaertner P, Bordry N, Cagnon L, Donda A, et al. Very late antigen-1 marks functional tumor-resident CD8 T cells and correlates with survival of melanoma patients. Front Immunol. 2016; Dec. 7:573.
Article30. Rau M, Schilling AK, Meertens J, Hering I, Weiss J, Jurowich C, et al. Progression from nonalcoholic fatty liver to nonalcoholic steatohepatitis is marked by a higher frequency of TH17 cells in the liver and an increased TH17/resting regulatory T cell ratio in peripheral blood and in the liver. J Immunol. 2016; Jan. 196(1):97–105.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of Superantigen in Nasal Polypogenesis
- Medical treatment according to phenotypes of chronic rhinosinusitis
- The Impact of Nasal Polyposis on Olfactory Dysfunction in Chronic Rhinosinusitis
- Update on Biologics in Treatment of Chronic Rhinosinusitis With Nasal Polyposis
- Prevalence of Subclinical Aspirin-Intolerant Asthma in Patients with Nasal Polyposis and Chronic Rhinosinusitis