J Vet Sci.
2016 Dec;17(4):505-513. 10.4142/jvs.2016.17.4.505.
Mycobacterium vaccae induces a strong Th1 response that subsequently declines in C57BL/6 mice
- Affiliations
-
- 1College of Animal Science and Technology, Jilin Agricultural University, Changchun 130118, China. wangchunfeng@jlau.edu.cn qianaidong0115@163.com
- 2College of Biological Science, Changchun Teacher University, Changchun 130032, China.
- KMID: 2412606
- DOI: http://doi.org/10.4142/jvs.2016.17.4.505
Abstract
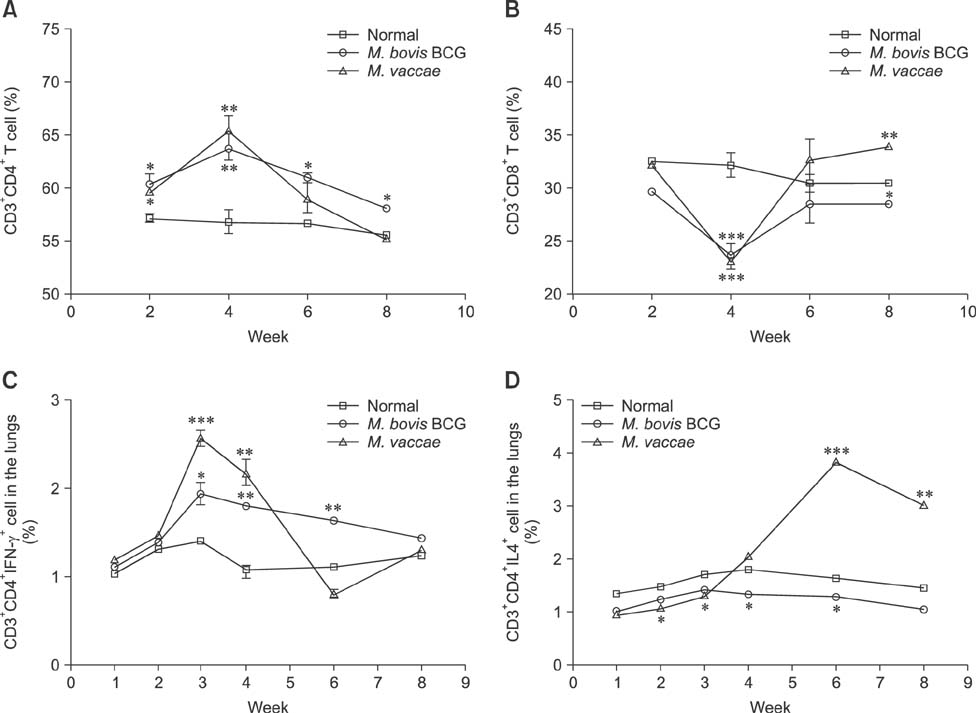
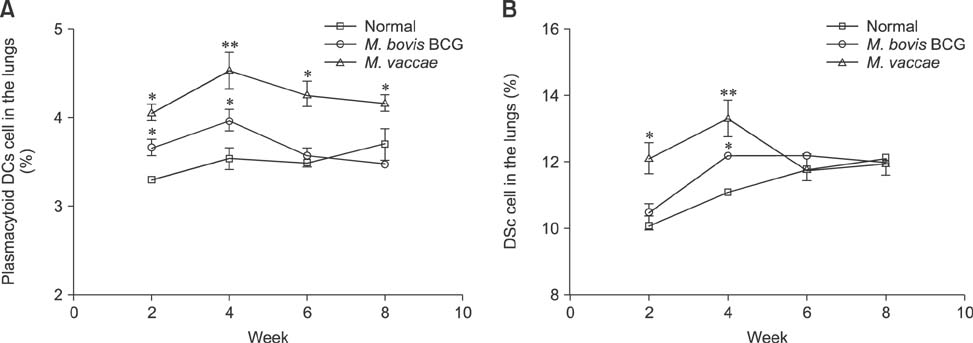
- Mycobacterium (M.) vaccae is a fast-growing species of saprophytic bacteria that is widely distributed. To understand the host immune responses induced by M. vaccae isolated from bovine submaxillary lymph nodes, C57BL/6 mice were infected with reference strain M. vaccae Bacillus Calmette-Guérin (BCG) and isolated M. vaccae using intraperitoneal injections. Comparison of the bacterial replication and organ pathology between M. vaccae and M. vaccae BCG revealed that M. vaccae was more malignant than M. vaccae in mice. We also demonstrated that serum from the M. vaccae-infected mice contained a higher expression level of gamma-interferon (IFN-γ), tumor necrosis factor alpha, monocyte chemoattractant protein-1, interleukin (IL)-4, IL-12, IL-10 and transforming growth factor beta than did the other groups, especially after week 4. Furthermore, when the numbers of CD3âºCD4âºIFN-γ⺠and CD3âºCD4âºIL4⺠cells in the infected mice were observed by flow cytometry, we found that a powerful T helper 1 (Th1) response was induced by M. vaccae infection, which was associated with the emergence of CD3âºCD4âºIFN-γ⺠cells. However, the Th1 response declined over time, which was associated with appearance of the CD4âºCD25âºFoxP3⺠and CD4âºCD25âºCD152âºTreg cell reaction. In addition, a strong Th2 response was found. Finally, we found that M. vaccae infection increased the production of type I IFNs, which was associated with a reduced Th1 response.
Keyword
MeSH Terms
Figure
Reference
-
1. Aksamit TR, Philley JV, Griffith DE. Nontuberculous mycobacterial (NTM) lung disease: the top ten essentials. Respir Med. 2014; 108:417–425.
Article2. Altare F, Durandy A, Lammas D, Emile JF, Lamhamedi S, Le Deist F, Drysdale P, Jouanguy E, Döffinger R, Bernaudin F, Jeppsson O, Gollob JA, Meinl E, Segal AW, Fischer A, Kumararatne D, Casanova JL. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science. 1998; 280:1432–1435.
Article3. Appelberg R, Castro AG, Pedrosa J, Silva RA, Orme IM, Minóprio P. Role of gamma interferon and tumor necrosis factor alpha during T-cell-independent and -dependent phases of Mycobacterium avium infection. Infect Immun. 1994; 62:3962–3971.
Article4. Arlotta A, Cefalo MG, Maurizi P, Ruggiero A, Dodi I, Riccardi R. Critical pulmonary infection due to nontuberculous mycobacterium in pediatric leukemia: report of a difficult diagnosis and review of pediatric series. J Pediatr Hematol Oncol. 2014; 36:66–70.
Article5. Asselin-Paturel C, Trinchieri G. Production of type I interferons: plasmacytoid dendritic cells and beyond. J Exp Med. 2005; 202:461–465.6. Bönicke R, Juhasz SE. Description of new species Mycobacterium vaccae no. sp. Zentralbl Bakteriol Parasitenkd Infekt Hyg. 1964; 192:133–135.7. Brown-Elliott BA, Wallace RJ Jr. Clinical and taxonomic status of pathogenic nonpigmented or late-pigmenting rapidly growing mycobacteria. Clin Microbiol Rev. 2002; 15:716–746.
Article8. Daley CL, Griffith DE. Pulmonary disease caused by rapidly growing mycobacteria. Clin Chest Med. 2002; 23:623–632. vii
Article9. Dheda K, Booth H, Huggett JF, Johnson MA, Zumla A, Rook GAW. Lung remodeling in pulmonary tuberculosis. J Infect Dis. 2005; 192:1201–1209.
Article10. Dorman SE, Picard C, Lammas D, Heyne K, van Dissel JT, Baretto R, Rosenzweig SD, Newport M, Levin M, Roesler J, Kumararatne D, Casanova JL, Holland SM. Clinical features of dominant and recessive interferon gamma receptor 1 deficiencies. Lancet. 2004; 364:2113–2121.
Article11. Egelund EF, Fennelly KP, Peloquin CA. Medications and monitoring in nontuberculous mycobacteria infections. Clin Chest Med. 2015; 36:55–66.
Article12. Feng CG, Jankovic D, Kullberg M, Cheever A, Scanga CA, Hieny S, Caspar P, Yap GS, Sher A. Maintenance of pulmonary Th1 effector function in chronic tuberculosis requires persistent IL-12 production. J Immunol. 2005; 174:4185–4192.
Article13. Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001; 19:93–129.
Article14. Gonzalez-Santiago TM, Drage LA. Nontuberculous mycobacteria: skin and soft tissue infections. Dermatol Clin. 2015; 33:563–577.15. Hambleton S, Salem S, Bustamante J, Bigley V, Boisson-Dupuis S, Azevedo J, Fortin A, Haniffa M, Ceron-Gutierrez L, Bacon CM, Menon G, Trouillet C, McDonald D, Carey P, Ginhoux F, Alsina L, Zumwalt TJ, Kong XF, Kumararatne D, Butler K, Hubeau M, Feinberg J, Al-Muhsen S, Cant A, Abel L, Chaussabel D, Doffinger R, Talesnik E, Grumach A, Duarte A, Abarca K, Moraes-Vasconcelos D, Burk D, Berghuis A, Geissmann F, Collin M, Casanova JL, Gros P. IRF8 mutations and human dendritic-cell immunodeficiency. N Engl J Med. 2011; 365:127–138.
Article16. Haug M, Awuh JA, Steigedal M, Frengen Kojen J, Marstad A, Nordrum IS, Halaas Ø, Flo TH. Dynamics of immune effector mechanisms during infection with Mycobacterium avium in C57BL/6 mice. Immunology. 2013; 140:232–243.
Article17. Henkle E, Winthrop KL. Nontuberculous mycobacteria infections in immunosuppressed hosts. Clin Chest Med. 2015; 36:91–99.
Article18. Hernandez-Pando R, Pavon L, Orozco EH, Rangel J, Rook GA. Interactions between hormone-mediated and vaccine-mediated immunotherapy for pulmonary tuberculosis in BALB/c mice. Immunology. 2000; 100:391–398.
Article19. Jönsson B, Ridell M, Wold AE. Non-tuberculous mycobacteria and their surface lipids efficiently induced IL-17 production in human T cells. Microbes Infect. 2012; 14:1186–1195.
Article20. Kobayashi T, Morino E, Takasaki J, Nagahara Y, Sugiyama H. Nontuberculous mycobacterial osteomyelitis in human immunodeficiency virus-negative patients: a case series. Jpn J Infect Dis. 2016; 69:149–150.
Article21. Koehne G, Maddux R, Britt J. Rapidly growing mycobacteria associated with bovine mastitis. Am J Vet Res. 1981; 42:1238–1239.22. Lienhardt C, Azzurri A, Amedei A, Fielding K, Sillah J, Sow OY, Bah B, Benagiano M, Diallo A, Manetti R, Manneh K, Gustafson P, Bennett S, D'Elios MM, McAdam K, Del Prete G. Active tuberculosis in Africa is associated with reduced Th1 and increased Th2 activity in vivo. Eur J Immunol. 2002; 32:1605–1613.
Article23. Monack DM, Mueller A, Falkow S. Persistent bacterial infections: the interface of the pathogen and the host immune system. Nat Rev Microbiol. 2004; 2:747–765.
Article24. Ordway D, Henao-Tamayo M, Harton M, Palanisamy G, Troudt J, Shanley C, Basaraba RJ, Orme IM. The hypervirulent Mycobacterium tuberculosis strain HN878 induces a potent Th1 response followed by rapid down-regulation. J Immunol. 2007; 179:522–531.
Article25. Ottenhoff TH, Verreck FAW, Hoeve MA, van de Vosse E. Control of human host immunity to mycobacteria. Tuberculosis (Edinb). 2005; 85:53–64.
Article26. Quinn KM, McHugh RS, Rich FJ, Goldsack LM, de Lisle GW, Buddle BM, Delahunt B, Kirman JR. Inactivation of CD4+ CD25+ regulatory T cells during early mycobacterial infection increases cytokine production but does not affect pathogen load. Immunol Cell Biol. 2006; 84:467–474.
Article27. Ramsdell F. Foxp3 and natural regulatory T cells: key to a cell lineage? Immunity. 2003; 19:165–168.28. Hachem R, Raad I, Rolston KVI, Whimbey E, Katz R, Tarrand J, Libshitz H. Cutaneous and pulmonary infections caused by Mycobacterium vaccae. Clin Infect Dis. 1996; 23:173–175.29. Rottman M, Catherinot E, Hochedez P, Emile JF, Casanova JL, Gaillard JL, Soudais C. Importance of T cells, gamma interferon, and tumor necrosis factor in immune control of the rapid grower Mycobacterium abscessus in C57BL/6 mice. Infect Immun. 2007; 75:5898–5907.
Article30. Seddiki N, Sasson SC, Santner-Nanan B, Munier M, van Bockel D, Ip S, Marriott D, Pett S, Nanan R, Cooper DA, Zaunders JJ, Kelleher AD. Proliferation of weakly suppressive regulatory CD4+ T cells is associated with over-active CD4+ T-cell responses in HIV-positive patients with mycobacterial immune restoration disease. Eur J Immunol. 2009; 39:391–403.
Article31. Shang S, Gibbs S, Henao-Tamayo M, Shanley CA, McDonnell G, Duarte RS, Ordway DJ, Jackson M. Increased virulence of an epidemic strain of Mycobacterium massiliense in mice. PLoS One. 2011; 6:e24726.32. Shimizu K, Hirose T, Sato M, Tsukamura M. Isolation of acid-fast organisms resembling Mycobacterium vaccae from a lesion of bovine nodular thelitis. Microbiol Immunol. 1977; 21:469–472.
Article33. Starke JR. Committee on Infectious Diseases. Interferon-γ release assays for diagnosis of tuberculosis infection and disease in children. Pediatrics. 2014; 134:e1763–e1773.34. Urdahl KB, Shafiani S, Ernst JD. Initiation and regulation of T-cell responses in tuberculosis. Mucosal Immunol. 2011; 4:288–293.
Article35. Velayati AA, Farnia P, Mozafari M, Mirsaeidi M. Nontuberculous mycobacteria isolation from clinical and environmental samples in Iran: twenty years of surveillance. Biomed Res Int. 2015; 2015:254285.
Article36. von Reyn CF, Mtei L, Arbeit RD, Waddell R, Cole B, Mackenzie T, Matee M, Bakari M, Tvaroha S, Adams LV, Horsburgh CR, Pallangyo K. DarDar Study Group. Prevention of tuberculosis in Bacille Calmette-Guerin-primed, HIV-infected adults boosted with an inactivated whole-cell mycobacterial vaccine. AIDS. 2010; 24:675–685.
Article37. Wang C, Qi H, Jiang X, Chen FF, Ma H, Wang C, Qian A. Molecular differentiation of nontuberculous Mycobacterium isolated from different animals. Appl Mech Mater. 2013; 421:300–303.
Article38. Wang CC, Rook GA. Inhibition of an established allergic response to ovalbumin in BALB/c mice by killed Mycobacterium vaccae. Immunology. 1998; 93:307–313.
Article39. Xu LJ, Wang Y, Zheng X, Gui X, Tao L, Wei H. Immunotherapeutical potential of Mycobacterium vaccae on M. tuberculosis infection in mice. Cell Mol Immunol. 2009; 6:67–72.
Article40. Zuany-Amorim C, Manlius C, Trifilieff A, Brunet LR, Rook G, Bowen G, Pay G, Walker C. Long-term protective and antigen-specific effect of heat-killed Mycobacterium vaccae in a murine model of allergic pulmonary inflammation. J Immunol. 2002; 169:1492–1499.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Concurrent Impairment of Th1 and Th2 Response in Thermal Injury
- BCG Immunomodulation Therapy in Asthma
- Inflammatory Skin Response to Ultraviolet Radiation: Ear Swelling Response in C57BL Mouse
- Concurrent response to challenge infection with Cryptosporidium parvum in immunosuppressed C57BL/6N mice
- In vitro Stimulation of Tumor - Draining Lymph Node Lymphocytes with the 30 kDa Antigen of Mycobacterium tuberculosis Leads to the Differentiation of Th1 Cells and Cytotoxic Effector Cells