J Vet Sci.
2017 Jun;18(2):149-158. 10.4142/jvs.2017.18.2.149.
Estrogen receptor-α, progesterone receptor, and c-erbB/HER-family receptor mRNA detection and phenotype analysis in spontaneous canine models of breast cancer
- Affiliations
-
- 1Auburn University Research Initiative in Cancer (AURIC), Department of Pathobiology, College of Veterinary Medicine, Harrison School of Pharmacy, Auburn University, AL 36849, USA. birdric@auburn.edu
- 2Auburn University Research Initiative in Cancer (AURIC), Scott-Ritchey Research Center, College of Veterinary Medicine, Harrison School of Pharmacy, Auburn University, AL 36849, USA.
- 3Auburn University Research Initiative in Cancer (AURIC), Department of Drug Discovery and Development, Harrison School of Pharmacy, Auburn University, AL 36849, USA.
- KMID: 2412567
- DOI: http://doi.org/10.4142/jvs.2017.18.2.149
Abstract
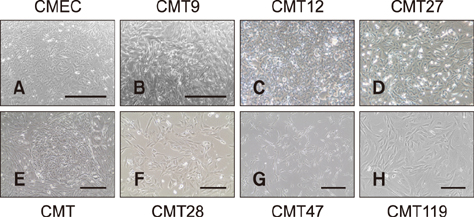
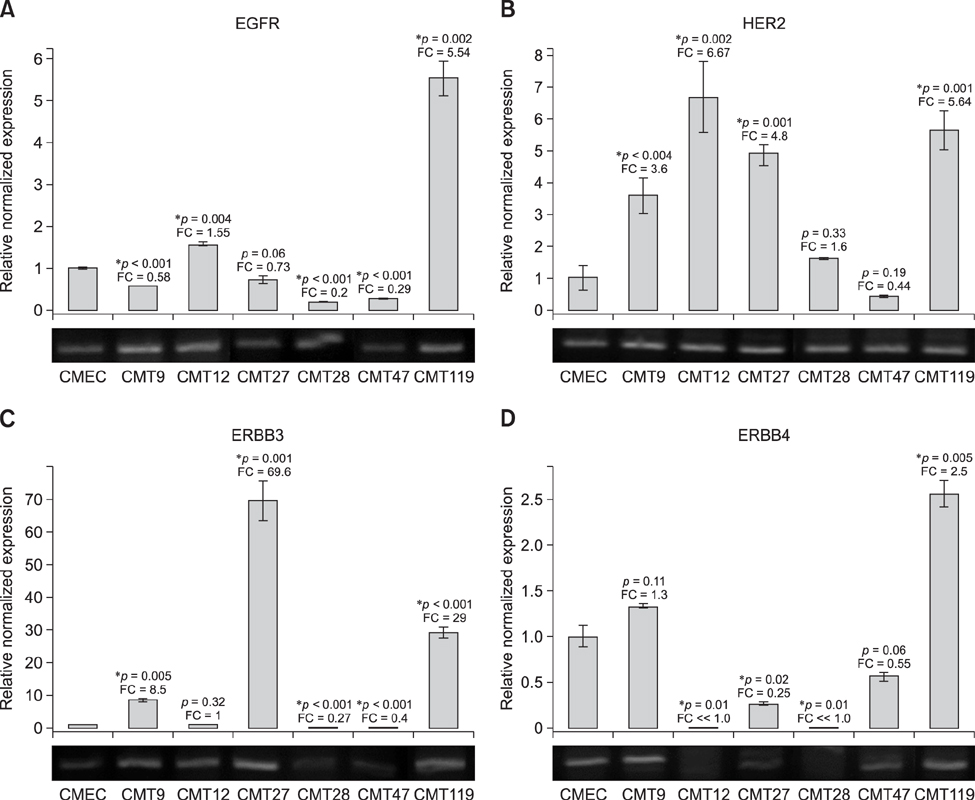
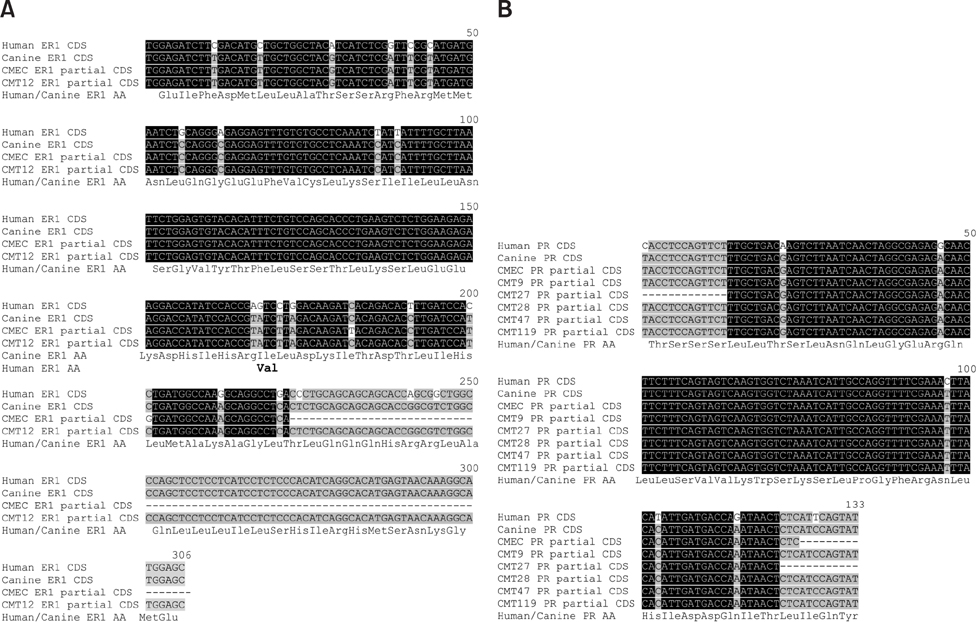
- Well characterized, stable, p16-defective canine mammary cancer (CMT) cell lines and normal canine mammary epithelial cells were used to investigate expression of the major breast cancer-specific hormone receptors estrogen receptor alpha (ER1) and progesterone receptor (PR) as well as luminal epithelial-specific proto-oncogenes encoding c-erbB-1 (epidermal growth factor receptor/EGFr), c-erbB-2/HER2, c-erbB-3, and c-erbB-4 receptors. The investigation developed and validated quantitative reverse transcriptase polymerase chain reaction assays for each transcript to provide rapid assessment of breast cancer phenotypes for canine cancers, based on ER1, PR, and c-erbB-2/HER2 expressions, similar to those in human disease. Roles for relatively underexplored c-erbB-3 and c-erbB-4 receptor expressions in each of these breast cancer phenotypes were also evaluated. Each quantitative assay was validated by assessment of amplicon size and DNA sequencing following amplification. Differential expression of ER1, PR, and c-erbB-2 in CMT cell lines clearly defined distinct human-like breast cancer phenotypes for a selection of CMT-derived cell lines. Expression profiles for EGFr family genes c-erbB-3 and c-erbB-4 in CMT models also provided an enriched classification of canine breast cancer identifying new extended phenotypes beyond the conventional luminal-basal characterization used in human breast cancer.
Keyword
MeSH Terms
-
Animals
Cell Line, Tumor
Disease Models, Animal
Dog Diseases/*metabolism
Dogs
ErbB Receptors/*metabolism
Estrogen Receptor alpha/*metabolism
Female
Mammary Neoplasms, Animal/*metabolism
Phenotype
Receptors, Progesterone/*metabolism
Reverse Transcriptase Polymerase Chain Reaction/veterinary
Estrogen Receptor alpha
Receptors, Progesterone
ErbB Receptors
Figure
Cited by 1 articles
-
Autologous hybrid cell fusion vaccine in a spontaneous intermediate model of breast carcinoma
R. Curtis Bird, Patricia DeInnocentes, Allison E. Church Bird, Farruk M. Lutful Kabir, E. Gisela Martinez-Romero, Annette N. Smith, Bruce F. Smith
J Vet Sci. 2019;20(5):. doi: 10.4142/jvs.2019.20.e48.
Reference
-
1. Ades F, Zardavas D, Bozovic-Spasojevic I, Pugliano L, Fumagalli D, de Azambuja E, Viale G, Sotiriou C, Piccart M. Luminal B breast cancer: molecular characterization, clinical management, and future perspectives. J Clin Oncol. 2014; 32:2794–2803.
Article2. Agarwal P, Sandey M, DeInnocentes P, Bird RC. Tumor suppressor gene p16/INK4A/CDKN2A-dependent regulation into and out of the cell cycle in a spontaneous canine model of breast cancer. J Cell Biochem. 2013; 114:1355–1363.
Article3. Ahern TE, Bird RC, Bird AEC, Wolfe LG. Expression of the oncogene c-erbB-2 in canine mammary cancers and tumor-derived cell lines. Am J Vet Res. 1996; 57:693–696.4. Arteaga CL, Engelman JA. ERBB receptors: from oncogene discovery to basic science to mechanism-based cancer therapeutics. Cancer Cell. 2014; 25:282–303.
Article5. Beck J, Hennecke S, Bornemann-Kolatzki K, Urnovitz HB, Neumann S, Ströbel P, Kaup FJ, Brenig B, Schütz E. Genome aberrations in canine mammary carcinomas and their detection in cell-free plasma DNA. PLoS One. 2013; 8:e75485.
Article6. Bird RC, Bird AEC, DeInnocentes P. Animal cell separation and subcellular fractionation. Encyclopedia of Life Sciences (ELS). Chichester: John Wiley & Sons;2011.7. Bird RC, DeInnocentes P, Bird AEC, van Ginkel FW, Lindquist J, Smith BF. An autologous dendritic cell canine mammary tumor hybrid-cell fusion vaccine. Cancer Immunol Immunother. 2011; 60:87–97.
Article8. Brenton JD, Carey LA, Ahmed AA, Caldas C. Molecular classification and molecular forecasting of breast cancer: ready for clinical application? J Clin Oncol. 2005; 23:7350–7360.
Article9. Burrai GP, Tanca A, De Miglio MR, Abbondio M, Pisanu S, Polinas M, Pirino S, Mohammed SI, Uzzau S, Addis MF, Antuofermo E. Investigation of HER2 expression in canine mammary tumors by antibody-based, transcriptomic and mass spectrometry analysis: is the dog a suitable animal model for human breast cancer? Tumour Biol. 2015; 36:9083–9091.
Article10. Caceres S, Peña L, de Andres PJ, Illera MJ, Lopez MS, Woodward WA, Reuben JM, Illera JC. Establishment and characterization of a new cell line of canine inflammatory mammary cancer: IPC-366. PLoS One. 2015; 10:e0122277.
Article11. Cullen JM, Page R, Misdorp W. An overview of cancer pathogenesis, diagnosis and management. In : Meuten DJ, editor. Tumors in Domestic Animals. 4th ed. Ames: Iowa State University Press;2002. p. 3–45.12. DeInnocentes P, Agarwal P, Bird RC. Phenotype-rescue of cyclin-dependent kinase inhibitor p16/INK4A defects in a spontaneous canine cell model of breast cancer. J Cell Biochem. 2009; 106:491–505.
Article13. DeInnocentes P, Li LX, Sanchez RL, Bird RC. Expression and sequence of canine SIRT2 and p53 genes in canine mammary tumour cells - effects on downstream targets Wip1 and p21/Cip1. Vet Comp Oncol. 2006; 4:161–177.
Article14. Gama A, Alves A, Schmitt F. Identification of molecular phenotypes in canine mammary carcinomas with clinical implications: application of the human classification. Virchows Arch. 2008; 453:123–132.
Article15. Goldschmidt M, Peña L, Rasotto R, Zappulli V. Classification and grading of canine mammary tumors. Vet Pathol. 2011; 48:117–131.
Article16. Im KS, Kim NH, Lim HY, Kim HW, Shin JI, Sur JH. Analysis of a new histological and molecular-based classification of canine mammary neoplasia. Vet Pathol. 2014; 51:549–559.
Article17. Kim NH, Lim HY, Im KS, Kim JH, Sur JH. Identification of triple-negative and basal-like canine mammary carcinomas using four basal markers. J Comp Pathol. 2013; 148:298–306.
Article18. Lutful Kabir FM, Agarwal P, DeInnocentes P, Zaman J, Bird AC, Bird RC. Novel frameshift mutation in the p16/INK4A tumor suppressor gene in canine breast cancer alters expression from the p16/INK4A/p14ARF locus. J Cell Biochem. 2013; 114:56–66.
Article19. Lutful Kabir FM, Alvarez CE, Bird RC. Canine mammary carcinomas: a comparative analysis of altered gene expression. Vet Sci. 2016; 3:1.
Article20. Lutful Kabir FM, DeInnocentes P, Bird RC. Altered microRNA expression profiles and regulation of INK4A/CDKN2A tumor suppressor genes in canine breast cancer models. J Cell Biochem. 2015; 116:2956–2969.
Article21. Onitilo AA, Engel JM, Greenlee RT. Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin Med Res. 2009; 7:4–13.
Article22. Peña L, Gama A, Goldschmidt MH, Abadie J, Benazzi C, Castagnaro M, Díez L, Gärtner F, Hellmén E, Kiupel M, Millán Y, Miller MA, Nguyen F, Poli A, Sarli G, Zappulli V, de las Mulas JM. Canine mammary tumors: a review and consensus of standard guidelines on epithelial and myoepithelial phenotype markers, HER2, and hormone receptor assessment using immunohistochemistry. Vet Pathol. 2014; 51:127–145.23. Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge Ø, Pergamenschikov A, Williams C, Zhu SX, Lønning PE, Børresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumours. Nature. 2000; 406:747–752.
Article24. Prat A, Parker JS, Karginova O, Fan C, Livasy C, Herschkowitz JI, He X, Perou CM. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 2010; 12:R68.
Article25. Sassi F, Benazzi C, Castellani G, Sarli G. Molecular-based tumour subtypes of canine mammary carcinomas assessed by immunohistochemistry. BMC Vet Res. 2010; 6:5.
Article26. Schiffman JD, Breen M. Comparative oncology: what dogs and other species can teach us about humans with cancer. Philos Trans R Soc Lond B Biol Sci. 2015; 370:20140231.
Article27. Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Lønning PE, Børresen-Dale AL. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001; 98:10869–10874.
Article28. Su S, Bird RC. Cell cycle, differentiation and tissue-independent expression of ribosomal protein L37. Eur J Biochem. 1995; 232:789–797.
Article29. Tonomura N, Elvers I, Thomas R, Megquier K, Turner-Maier J, Howald C, Sarver AL, Swofford R, Frantz AM, Ito D, Mauceli E, Arendt M, Noh HJ, Koltookian M, Biagi T, Fryc S, Williams C, Avery AC, Kim JH, Barber L, Burgess K, Lander ES, Karlsson EK, Azuma C, Modiano JF, Breen M, Lindblad-Toh K. Genome-wide association study identifies shared risk loci common to two malignancies in golden retrievers. PLoS Genet. 2015; 11:e1004922.
Article30. Wolfe LG, Smith BB, Toivio-Kinnucan MA, Sartin EA, Kwapien RP, Henderson RA, Barnes S. Biologic properties of cell lines derived from canine mammary carcinomas. J Natl Cancer Inst. 1986; 77:783–792.
Article31. You J, Bird RC. Selective induction of cell cycle regulatory genes cdk1 (p34cdc2), cyclins A/B, and the tumor suppressor gene Rb in transformed cells by okadaic acid. J Cell Physiol. 1995; 164:424–433.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunohistochemical Expression of c-erbB2, c-erbB3 and c-erbB4 Protein in Breast Cancer
- Patients with Concordant Triple-Negative Phenotype between Primary Breast Cancers and Corresponding Metastases Have Poor Prognosis
- Relation Between Hormone Receptor (Enzyme-Immunoassay and Immunohistochemistry), Histologic Grade and Mammographic Findings in Patients with Primary Breast Cancer
- A Case of Estrogen and Progesterone Receptor-Positive Male Breast Cancer
- The Effect of Tamoxifen of the Estrogen Receptor cDNA-Iipofected MDA-MB-231 Human Breast Cancer Cells