J Breast Cancer.
2016 Sep;19(3):268-274. 10.4048/jbc.2016.19.3.268.
Patients with Concordant Triple-Negative Phenotype between Primary Breast Cancers and Corresponding Metastases Have Poor Prognosis
- Affiliations
-
- 1Department of Surgery, Chung-Ang University Hospital, Seoul, Korea.
- 2Department of Surgery, Seoul National University Hospital, Seoul, Korea. dynoh@snu.ac.kr
- 3Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- 4Department of Pathology, Seoul National University Hospital, Seoul, Korea.
- KMID: 2413951
- DOI: http://doi.org/10.4048/jbc.2016.19.3.268
Abstract
- PURPOSE
We investigated the prognostic impact of discordance between the receptor status of primary breast cancers and corresponding metastases.
METHODS
A total 144 patients with breast cancer and distant metastasis were investigated. The estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) status of primary tumor and corresponding metastases were assessed. Tumor phenotype according to receptor status was classified as triple-negative phenotype (TNP) or non-TNP. Concordance and discordance was determined by whether there was a change in receptor status or phenotype between primary and metastatic lesions.
RESULTS
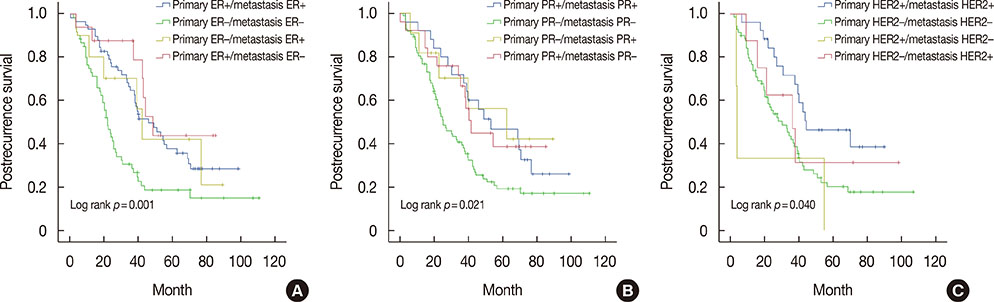
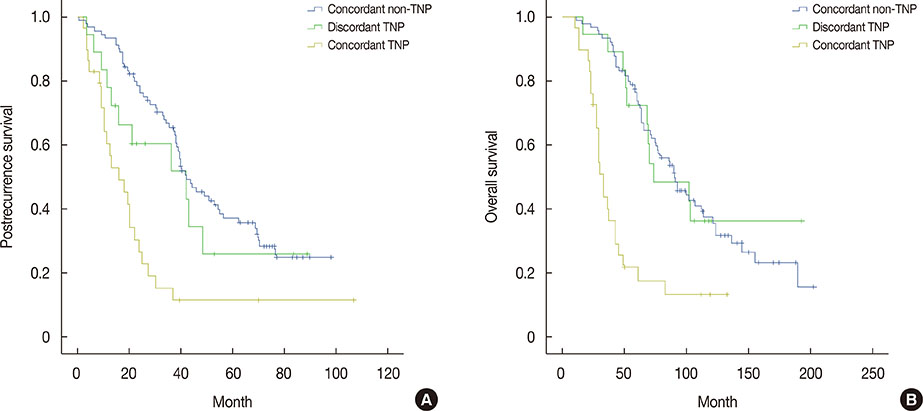
The rates of discordance between primary breast cancer and metastatic lesions were 18.1%, 25.0%, and 10.3% for ER, PR, and HER2, respectively. The rates of concordant non-TNP, concordant TNP and discordant TNP were 65.9%, 20.9%, and 13.2%, respectively. Patients with concordant ER/PR-negative status had worse postrecurrence survival (PRS) than patients with concordant ER/PR-positive and discordant ER/PR status (p=0.001 and p=0.021, respectively). Patients who converted from HER2-positive to negative after distant metastasis had worst PRS (p=0.040). Multivariate analysis showed that concordant TNP was statistically significant factor for worse PRS (p<0.001).
CONCLUSION
Discordance in receptor status and tumor phenotype between primary breast cancer and corresponding metastatic lesions was observed. Patients with concordant TNP had worse long-term outcomes than patients with concordant non-TNP and discordant TNP between primary and metastatic breast cancer. Identifying the receptor status of metastatic lesions may lead to improvements in patient management and survival.
MeSH Terms
Figure
Cited by 2 articles
-
Comment to “Patients with Concordant Triple-Negative Phenotype between Primary Breast Cancers and Corresponding Metastases Have Poor Prognosis”
Kadri Altundag
J Breast Cancer. 2016;19(4):465-465. doi: 10.4048/jbc.2016.19.4.465.Comment to “Patients with Concordant Triple-Negative Phenotype between Primary Breast Cancers and Corresponding Metastases Have Poor Prognosis”
Kadri Altundag
J Breast Cancer. 2016;19(4):465-465. doi: 10.4048/jbc.2016.19.4.465.
Reference
-
1. Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000; 406:747–752.
Article2. Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001; 98:10869–10874.
Article3. Harris JR, Lippman ME, Morrow M, Osborne CK. Diseases of the Breast. 4th ed. Philadelphia: Lippincott Williams & Wilkins;2010.4. NCCN clinical practice guidelines in oncology: breast cancer v. 1. National Comprehensive Cancer Network;2013. Accessed July 7th, 2016. http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.5. Guarneri V, Giovannelli S, Ficarra G, Bettelli S, Maiorana A, Piacentini F, et al. Comparison of HER-2 and hormone receptor expression in primary breast cancers and asynchronous paired metastases: impact on patient management. Oncologist. 2008; 13:838–844.
Article6. Broom RJ, Tang PA, Simmons C, Bordeleau L, Mulligan AM, O'Malley FP, et al. Changes in estrogen receptor, progesterone receptor and Her-2/neu status with time: discordance rates between primary and metastatic breast cancer. Anticancer Res. 2009; 29:1557–1562.7. Lindström LS, Karlsson E, Wilking UM, Johansson U, Hartman J, Lidbrink EK, et al. Clinically used breast cancer markers such as estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 are unstable throughout tumor progression. J Clin Oncol. 2012; 30:2601–2608.
Article8. Pérez G, Aranda C, Olivares IM, García JR. Prevalence of hormonal receptors and human epidermal growth factor receptor 2 in Mexican female patients with breast cancer. Med Clin (Barc). 2014; 143:231–232.
Article9. Idirisinghe PK, Thike AA, Cheok PY, Tse GM, Lui PC, Fook-Chong S, et al. Hormone receptor and c-ERBB2 status in distant metastatic and locally recurrent breast cancer. Pathologic correlations and clinical significance. Am J Clin Pathol. 2010; 133:416–429.
Article10. Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013; 31:3997–4013.
Article11. Ibrahim T, Farolfi A, Scarpi E, Mercatali L, Medri L, Ricci M, et al. Hormonal receptor, human epidermal growth factor receptor-2, and Ki67 discordance between primary breast cancer and paired metastases: clinical impact. Oncology. 2013; 84:150–157.
Article12. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977; 33:159–174.
Article13. Niikura N, Liu J, Hayashi N, Mittendorf EA, Gong Y, Palla SL, et al. Loss of human epidermal growth factor receptor 2 (HER2) expression in metastatic sites of HER2-overexpressing primary breast tumors. J Clin Oncol. 2012; 30:593–599.
Article14. Matsumoto A, Jinno H, Murata T, Seki T, Takahashi M, Hayashida T, et al. Prognostic implications of receptor discordance between primary and recurrent breast cancer. Int J Clin Oncol. 2015; 20:701–708.
Article15. Rossi S, Basso M, Strippoli A, Dadduzio V, Cerchiaro E, Barile R, et al. Hormone receptor status and HER2 expression in primary breast cancer compared with synchronous axillary metastases or recurrent metastatic disease. Clin Breast Cancer. 2015; 15:307–312.
Article16. Dieci MV, Barbieri E, Piacentini F, Ficarra G, Bettelli S, Dominici M, et al. Discordance in receptor status between primary and recurrent breast cancer has a prognostic impact: a single-institution analysis. Ann Oncol. 2013; 24:101–108.
Article17. Lower EE, Glass E, Blau R, Harman S. HER-2/neu expression in primary and metastatic breast cancer. Breast Cancer Res Treat. 2009; 113:301–306.
Article18. Liedtke C, Broglio K, Moulder S, Hsu L, Kau SW, Symmans WF, et al. Prognostic impact of discordance between triple-receptor measurements in primary and recurrent breast cancer. Ann Oncol. 2009; 20:1953–1958.
Article19. Chen S, Chen CM, Yu KD, Zhou RJ, Shao ZM. Prognostic value of a positive-to-negative change in hormone receptor status after neoadjuvant chemotherapy in patients with hormone receptor-positive breast cancer. Ann Surg Oncol. 2012; 19:3002–3011.
Article20. Guarneri V, Dieci MV, Barbieri E, Piacentini F, Omarini C, Ficarra G, et al. Loss of HER2 positivity and prognosis after neoadjuvant therapy in HER2-positive breast cancer patients. Ann Oncol. 2013; 24:2990–2994.
Article21. Wu JM, Fackler MJ, Halushka MK, Molavi DW, Taylor ME, Teo WW, et al. Heterogeneity of breast cancer metastases: comparison of therapeutic target expression and promoter methylation between primary tumors and their multifocal metastases. Clin Cancer Res. 2008; 14:1938–1946.
Article22. Mittendorf EA, Wu Y, Scaltriti M, Meric-Bernstam F, Hunt KK, Dawood S, et al. Loss of HER2 amplification following trastuzumab-based neoadjuvant systemic therapy and survival outcomes. Clin Cancer Res. 2009; 15:7381–7388.
Article23. Shah SP, Morin RD, Khattra J, Prentice L, Pugh T, Burleigh A, et al. Mutational evolution in a lobular breast tumour profiled at single nucleotide resolution. Nature. 2009; 461:809–813.
Article24. Gong Y, Symmans WF, Krishnamurthy S, Patel S, Sneige N. Optimal fixation conditions for immunocytochemical analysis of estrogen receptor in cytologic specimens of breast carcinoma. Cancer. 2004; 102:34–40.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comment to “Patients with Concordant Triple-Negative Phenotype between Primary Breast Cancers and Corresponding Metastases Have Poor Prognosisâ€
- Clinicopathologic Characteristics and Prognosis of Early Stage Triple Negative Breast Cancer: Comparison with Non-triple Negative Group
- Overexpression of Cell Cycle Progression Inhibitor Geminin is Associated with Tumor Stem-Like Phenotype of Triple-Negative Breast Cancer
- Predictive Factors and Survival Rate for Brain Metastasis from Breast Cancer
- Ultrasound and Clinicopathological Characteristics of Triple Receptor-Negative Breast Cancers