J Vet Sci.
2017 Jun;18(2):141-148. 10.4142/jvs.2017.18.2.141.
Effect of donor age on the proliferation and multipotency of canine adipose-derived mesenchymal stem cells
- Affiliations
-
- 1Viral Disease Research Division, Animal and Plant Quarantine Agency, Gimcheon 39660, Korea. virusmania@korea.kr
- KMID: 2412566
- DOI: http://doi.org/10.4142/jvs.2017.18.2.141
Abstract
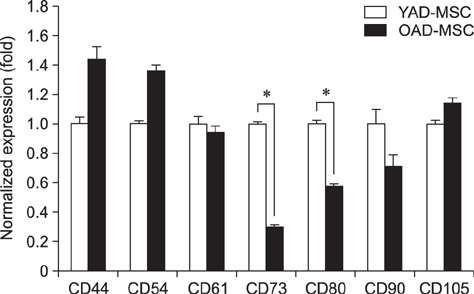
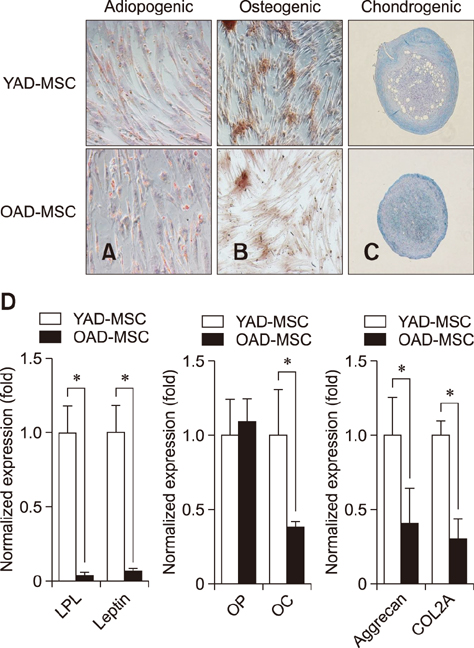
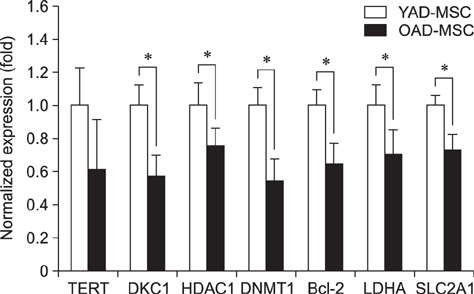
- Research into adipose tissue-derived mesenchymal stem cells (AD-MSCs) has demonstrated the feasibility of their use in clinical applications due to their ease of isolation and abundance in adipose tissue. We isolated AD-MSCs from young and old dogs, and the cells were subjected to sequential sub-passaging from passage 1 (P1) to P7. Canine AD-MSCs (cAD-MSCs) were examined for proliferation kinetics, expression of molecules associated with self-renewal, expression of cell surface markers, and differentiation potentials at P3. Cumulative population doubling level was significantly higher in cAD-MSCs of young donors than in those of old donors. In addition, expressions of CD73, CD80, Oct3/4, Nanog, cell survival genes and differentiation potentials were significantly higher in young donors than in old donors. The present study suggests that donor age should be considered when developing cell-based therapies for clinical application of cAD-MSCs.
MeSH Terms
Figure
Reference
-
1. Alt EU, Senst C, Murthy SN, Slakey DP, Dupin CL, Chaffin AE, Kadowitz PJ, Izadpanah R. Aging alters tissue resident mesenchymal stem cells properties. Stem Cell Res. 2012; 8:215–225.2. Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007; 25:2739–2749.
Article3. Choudhery MS, Khan M, Mahmood R, Mehmood A, Khan SN, Riazuddin S. Bone marrow derived mesenchymal stem cells from aged mice have reduced wound healing, angiogenesis, proliferation and anti-apoptosis capabilities. Cell Biol Int. 2012; 36:747–753.
Article4. Cristofalo VJ, Allen RG, Pignolo RJ, Martin BG, Beck JC. Relationship between donor age and the replicative lifespan of human cells in culture: a reevaluation. Proc Natl Acad Sci U S A. 1998; 95:10614–10619.
Article5. Feng X, Xing J, Feng G, Sang A, Shen B, Xu Y, Jiang J, Liu S, Tan W, Gu Z, Li L. Age dependent impaired neurogenic differentiation capacity of dental stem cell is associated with Wnt/XMLLink_XYZ-catenin signaling. Cell Mol Neurobiol. 2013; 33:1023–1031.
Article6. Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006; 24:150–154.
Article7. Gimble J, Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotheraphy. 2003; 5:362–369.
Article8. Guercio A, Di Bella S, Casella S, Di Marco P, Russo C, Piccione G. Canine mesenchymal stem cells (MSCs): characterization in relation to donor age and adipose tissue-harvesting site. Cell Biol Int. 2013; 37:789–798.
Article9. Iohara K, Murakami M, Nakata K, Nakashima M. Age-dependent decline in dental pulp regeneration after pulpectomy in dogs. Exp Gerontol. 2014; 52:39–45.
Article10. Itahana Y, Han R, Barbier S, Lei Z, Rozen S, Itahana K. The uric acid transporter SLC2A9 is a direct target gene of the tumor suppressor p53 contributing to antioxidant defense. Oncogene. 2015; 34:1799–1810.
Article11. Kanawa M, Igarashi A, Ronald VS, Higashi Y, Kurihara H, Sugiyama M, Saskianti T, Pan H, Kato Y. Age-dependent decrease in the chondrogenic potential of human bone marrow mesenchymal stromal cells expanded with fibroblast growth factor-2. Cytotherapy. 2013; 15:1062–1072.
Article12. Kretlow JD, Jin YQ, Liu W, Zhang WJ, Hong TH, Zhou G, Baggett LS, Mikos AG, Cao Y. Donor age and cell passage affects differentiation potential of murine bone marrow-derived stem cells. BMC Cell Biol. 2008; 9:60.
Article13. Lee KS, Cha SH, Kang HW, Song JY, Lee KW, Ko KB, Lee HT. Effects of serial passage on the characteristics and chondrogenic differentiation of canine umbilical cord matrix derived mesenchymal stem cells. Asian-Australas J Anim Sc. 2013; 26:588–595.
Article14. Lee KS, Kang HW, Lee HT, Kim HJ, Kim CL, Song JY, Lee KW, Cha SH. Sequential sub-passage decreases the differentiation potential of canine adipose-derived mesenchymal stem cells. Res Vet Sci. 2014; 96:267–275.
Article15. Lee KS, Nah JJ, Lee BC, Lee HT, Lee HS, So BJ, Cha SH. Maintenance and characterization of multipotent mesenchymal stem cells isolated from canine umbilical cord matrix by collagenase digestion. Res Vet Sci. 2013; 94:144–151.
Article16. Li Q, Qi LJ, Guo ZK, Li H, Zuo HB, Li NN. CD73+ adipose-derived mesenchymal stem cells possess higher potential to differentiate into cardiomyocytes in vitro. J Mol Histol. 2013; 44:411–422.
Article17. Li Y, Charif N, Mainard D, Bensoussan D, Stoltz JF, de Isla N. Donor's age dependent proliferation decrease of human bone marrow mesenchymal stem cells is linked to diminished clonogenicity. Biomed Mater Eng. 2014; 24:Suppl 1. 47–52.
Article18. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods. 2001; 25:402–408.
Article19. Mizuno H. Adipose-derived stem cells for tissue repair and regeneration: ten years of research and a literature review. J Nippon Med Sch. 2009; 76:55–66.
Article20. Neupane M, Chang CC, Kiupel M, Yuzbasiyan-Gurkan V. Isolation and characterization of canine adipose-derived mesenchymal stem cells. Tissue Eng Part A. 2008; 14:1007–1015.
Article21. Ode A, Kopf J, Kurtz A, Schmidt-Bleek K, Schrade P, Kolar P, Buttgereit F, Lehmann K, Hutmacher DW, Duda GN, Kasper G. CD73 and CD29 concurrently mediate the mechanically induced decrease of migratory capacity of mesenchymal stromal cells. Eur Cell Mater. 2011; 6:26–42.
Article22. Oh YS, Jeong SG, Cho GW. Anti-senescence effects of DNA methyltransferase inhibitor RG108 in human bone marrow mesenchymal stromal cells. Biotechnol Appl Biochem. 2015; 62:583–590.
Article23. Ohsaki H, Sawa T, Sasazaki S, Kano K, Taniguchi M, Mukai F, Mannen H. Stearoyl-CoA desaturase mRNA expression during bovine adipocyte differentiation in primary culture derived from Japanese Black and Holstein cattle. Comp Biochem Physiol A Mol Integr Physiol. 2007; 148:629–634.
Article24. Paxson JA, Gruntman AM, Davis AM, Parkin CM, Ingenito EP, Hoffman AM. Age dependence of lung mesenchymal stromal cell dynamics following pneumonectomy. Stem Cells Dev. 2013; 22:3214–3225.
Article25. Requicha JF, Viegas CA, Albuquerque CM, Azevedo JM, Reis RL, Gomes ME. Effect of anatomical origin and cell passage number on the stemness and osteogenic differentiation potential of canine adipose-derived stem cells. Stem Cell Rev. 2012; 8:1211–1222.
Article26. Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008; 129:163–173.
Article27. Takemitsu H, Zhao D, Yamamoto I, Harada Y, Michishita M, Arai T. Comparison of bone marrow and adipose tissue-derived canine mesenchymal stem cells. BMC Vet Res. 2012; 8:150.
Article28. Vaags AK, Rosic-Kablar S, Gartley CJ, Zheng YZ, Chesney A, Villagómez DAF, Kruth SA, Hough MR. Derivation and characterization of canine embryonic stem cell lines with in vitro and in vivo differentiation potential. Stem Cells. 2009; 27:329–340.
Article29. Vieira NM, Brandalise V, Zucconi E, Secco M, Strauss BE, Zatz M. Isolation, characterization, and differentiation potential of canine adipose-derived stem cells. Cell Transplant. 2010; 19:279–289.
Article30. Wagner W, Bork S, Horn P, Krunic D, Walenda T, Diehlmann A, Benes V, Blake J, Huber FX, Eckstein V, Boukamp P, Ho AD. Aging and replicative senescence have related effects on human stem and progenitor cells. PLoS One. 2009; 4:e5846.
Article31. Wang YH, Israelsen WJ, Lee D, Yu VWC, Jeanson NT, Clish CB, Cantley LC, Vander Heiden MG, Scadden DT. Cell-state-specific metabolic dependency in hematopoiesis and leukemogenesis. Cell. 2014; 158:1309–1323.
Article32. Wenceslau CV, Miglino MA, Martins DS, Ambrósio CE, Lizier NF, Pignatari GC, Kerkis I. Mesenchymal progenitor cells from canine fetal tissues: yolk sac, liver, and bone marrow. Tissue Eng Part A. 2011; 17:2165–2176.
Article33. Yang CC, Ellis SE, Xu F, Burg KJL. In vitro regulation of adipogenesis: tunable engineered tissues. J Tissue Eng Regen Med. 2007; 1:146–153.
Article34. Zheng B, Cao B, Li G, Huard J. Mouse adipose-derived stem cells undergo multilineage differentiation in vitro but primarily osteogenic and chondrogenic differentiation in vivo. Tissue Eng. 2006; 12:1891–1901.
Article35. Zhou S, Greenberger JS, Epperly MW, Goff JP, Adler C, Leboff MS, Glowacki J. Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell. 2008; 7:335–343.
Article36. Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002; 13:4279–4295.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Stem cell properties of cells derived from canine periodontal ligament
- Chondrogenesis of Mesenchymal Stem Cell Derived from Canine Adipose Tissue
- Characterization of multipotent mesenchymal stem cells isolated from adipose tissue and bone marrow in pigs
- Nervonic Acid Inhibits Replicative Senescence of Human Wharton’s Jelly-Derived Mesenchymal Stem Cells
- Flow cytometric immunophenotyping of canine adipose-derived mesenchymal stem cells (ADMSCs) and feline ADMSCs using anti-human antibodies