Yonsei Med J.
2018 Jun;59(4):463-469. 10.3349/ymj.2018.59.4.463.
Moonlighting Activity of Secreted Inflammation-Regulatory Proteins
- Affiliations
-
- 1Department of Otorhinolaryngology, Yonsei University College of Medicine, Seoul, Korea.
- 2Department of Otolaryngology-Head and Neck Surgery, Center for Thyroid Cancer, Research Institute and Hospital, National Cancer Center, Goyang, Korea.
- 3Laboratory of Signal Transduction, College of Veterinary Medicine and Research Institute for Veterinary Science, Seoul National University, Seoul, Korea. baeksj@snu.ac.kr
- KMID: 2410900
- DOI: http://doi.org/10.3349/ymj.2018.59.4.463
Abstract
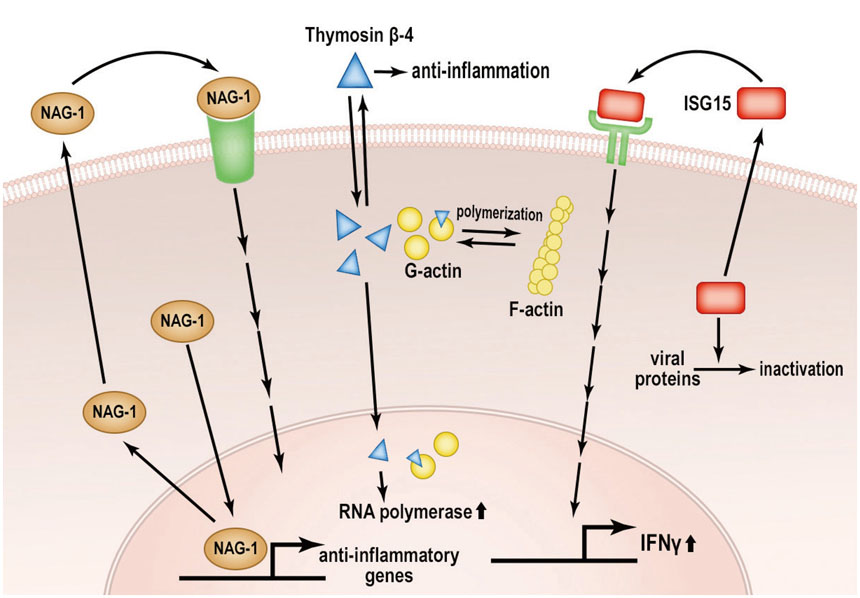
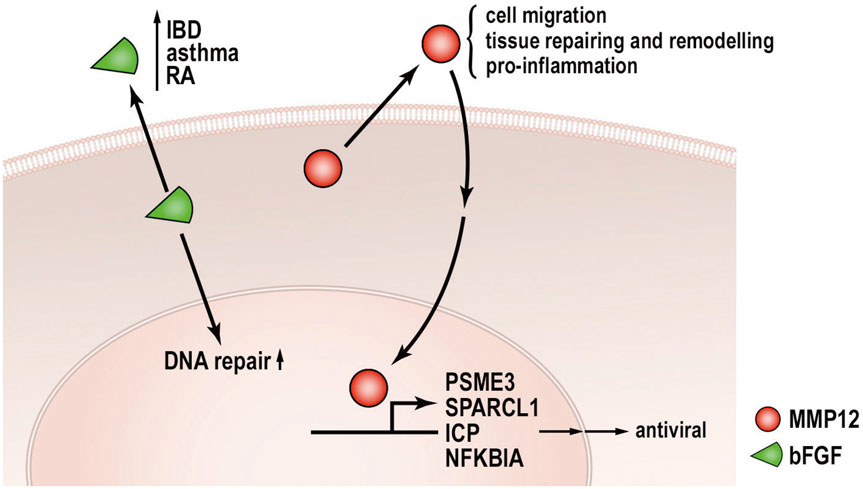
- Moonlighting proteins exhibit multiple activities in different cellular compartments, and their abnormal regulation could play an important role in many diseases. To date, many proteins have been identified with moonlighting activity, and more such proteins are being gradually identified. Among the proteins that possess moonlighting activity, several secreted proteins exhibit multiple activities in different cellular locations, such as the extracellular matrix, nucleus, and cytoplasm. While acute inflammation starts rapidly and generally disappears in a few days, chronic inflammation can last for months or years. This is generally because of the failure to eliminate the cause of inflammation, along with repeated exposure to the inflammatory agent. Chronic inflammation is now considered as an overwhelming burden to the general wellbeing of patients and noted as an underlying cause of several diseases. Moonlighting proteins can contribute to the process of chronic inflammation; therefore, it is imperative to overview some proteins that exhibit multiple functions in inflammatory diseases. In this review, we will focus on inflammation, particularly unravelling several well-known secreted proteins with multiple functions in different cellular locations.
Figure
Reference
-
1. Smith LM, Kelleher NL. Consortium for Top Down Proteomics. Proteoform: a single term describing protein complexity. Nat Methods. 2013; 10:186–187.
Article2. Muñoz J, Heck AJ. From the human genome to the human proteome. Angew Chem Int Ed Engl. 2014; 53:10864–10866.
Article3. Jeffery CJ. Moonlighting proteins--an update. Mol Biosyst. 2009; 5:345–350.
Article4. Copley SD. Moonlighting is mainstream: paradigm adjustment required. Bioessays. 2012; 34:578–588.
Article5. Henderson B, Martin A. Bacterial virulence in the moonlight: multitasking bacterial moonlighting proteins are virulence determinants in infectious disease. Infect Immun. 2011; 79:3476–3491.
Article6. Kainulainen V, Korhonen TK. Dancing to another tune-adhesive moonlighting proteins in bacteria. Biology (Basel). 2014; 3:178–204.
Article7. Min KW, Lee SH, Baek SJ. Moonlighting proteins in cancer. Cancer Lett. 2016; 370:108–116.
Article8. Shacter E, Weitzman SA. Chronic inflammation and cancer. Oncology (Williston Park). 2002; 16:217–226.9. Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, et al. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000; 21:383–421.
Article10. Kuzan A. Thymosin β as an actin-binding protein with a variety of functions. Adv Clin Exp Med. 2016; 25:1331–1336.
Article11. Jain AK, Moore SM, Yamaguchi K, Eling TE, Baek SJ. Selective nonsteroidal anti-inflammatory drugs induce thymosin beta-4 and alter actin cytoskeletal organization in human colorectal cancer cells. J Pharmacol Exp Ther. 2004; 311:885–891.
Article12. Lee SI, Yi JK, Bae WJ, Lee S, Cha HJ, Kim EC. Thymosin beta-4 suppresses osteoclastic differentiation and inflammatory responses in human periodontal ligament cells. PLoS One. 2016; 11:e0146708.
Article13. Conte E, Genovese T, Gili E, Esposito E, Iemmolo M, Fruciano M, et al. Protective effects of thymosin β4 in a mouse model of lung fibrosis. Ann N Y Acad Sci. 2012; 1269:69–73.
Article14. Bao W, Ballard VL, Needle S, Hoang B, Lenhard SC, Tunstead JR. . Cardioprotection by systemic dosing of thymosin beta four following ischemic myocardial injury. Front Pharmacol. 2013; 4:149.
Article15. Bock-Marquette I, Saxena A, White MD, Dimaio JM, Srivastava D. Thymosin beta4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature. 2004; 432:466–472.
Article16. Goldstein AL, Kleinman HK. Advances in the basic and clinical applications of thymosin β4. Expert Opin Biol Ther. 2015; 15:Suppl 1. S139–S145.
Article17. Piludu M, Piras M, Pichiri G, Coni P, Orrù G, Cabras T, et al. Thymosin beta 4 may translocate from the cytoplasm in to the nucleus in HepG2 cells following serum starvation. An ultrastructural study. PLoS One. 2015; 10:e0119642.
Article18. Huff T, Rosorius O, Otto AM, Müller CS, Ballweber E, Hannappel E, et al. Nuclear localisation of the G-actin sequestering peptide thymosin beta4. J Cell Sci. 2004; 117(Pt 22):5333–5341.19. Santra M, Zhang ZG, Yang J, Santra S, Chopp M, et al. Thymosin β4 up-regulation of microRNA-146a promotes oligodendrocyte differentiation and suppression of the Toll-like proin flammatory pathway. J Biol Chem. 2014; 289:19508–19518.
Article20. Shen XZ, Bernstein KE. The peptide network regulated by angiotensin converting enzyme (ACE) in hematopoiesis. Cell Cycle. 2011; 10:1363–1369.
Article21. Eling TE, Baek SJ, Shim M, Lee CH. NSAID activated gene (NAG-1), a modulator of tumorigenesis. J Biochem Mol Biol. 2006; 39:649–655.
Article22. Chrysovergis K, Wang X, Kosak J, Lee SH, Kim JS, Foley JF, et al. NAG-1/GDF-15 prevents obesity by increasing thermogenesis, lipolysis and oxidative metabolism. Int J Obes (Lond). 2014; 38:1555–1564.
Article23. Bootcov MR, Bauskin AR, Valenzuela SM, Moore AG, Bansal M, He XY, et al. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc Natl Acad Sci U S A. 1997; 94:11514–11519.
Article24. Detmer K, Steele TA, Shoop MA, Dannawi H. Lineage-restricted expression of bone morphogenetic protein genes in human hematopoietic cell lines. Blood Cells Mol Dis. 1999; 25:310–323.
Article25. Baek SJ, Kim JS, Moore SM, Lee SH, Martinez J, Eling TE. Cyclooxygenase inhibitors induce the expression of the tumor suppressor gene EGR-1, which results in the up-regulation of NAG-1, an antitumorigenic protein. Mol Pharmacol. 2005; 67:356–364.
Article26. Baek SJ, Kim KS, Nixon JB, Wilson LC, Eling TE. Cyclooxygenase inhibitors regulate the expression of a TGF-beta superfamily member that has proapoptotic and antitumorigenic activities. Mol Pharmacol. 2001; 59:901–908.
Article27. Baek SJ, Wilson LC, Eling TE. Resveratrol enhances the expression of non-steroidal anti-inflammatory drug-activated gene (NAG-1) by increasing the expression of p53. Carcinogenesis. 2002; 23:425–434.
Article28. Li PX, Wong J, Ayed A, Ngo D, Brade AM, Arrowsmith C, et al. Placental transforming growth factor-beta is a downstream mediator of the growth arrest and apoptotic response of tumor cells to DNA damage and p53 overexpression. J Biol Chem. 2000; 275:20127–20135.
Article29. Tan M, Wang Y, Guan K, Sun Y. PTGF-beta, a type beta transforming growth factor (TGF-beta) superfamily member, is a p53 target gene that inhibits tumor cell growth via TGF-beta signaling pathway. Proc Natl Acad Sci U S A. 2000; 97:109–114.
Article30. Cekanova M, Lee SH, Donnell RL, Sukhthankar M, Eling TE, Fischer SM, et al. Nonsteroidal anti-inflammatory drug-activated gene-1 expression inhibits urethane-induced pulmonary tumorigenesis in transgenic mice. Cancer Prev Res (Phila). 2009; 2:450–458.
Article31. Kim JM, Kosak JP, Kim JK, Kissling G, Germolec DR, Zeldin DC, et al. NAG-1/GDF15 transgenic mouse has less white adipose tissue and a reduced inflammatory response. Mediators Inflamm. 2013; 2013:641851.
Article32. Min KW, Liggett JL, Silva G, Wu WW, Wang R, Shen RF, et al. NAG-1/GDF15 accumulates in the nucleus and modulates transcriptional regulation of the Smad pathway. Oncogene. 2016; 35:377–388.
Article33. Emmerson PJ, Wang F, Du Y, Liu Q, Pickard RT, Gonciarz MD, et al. The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nat Med. 2017; 23:1215–1219.
Article34. Hsu JY, Crawley S, Chen M, Ayupova DA, Lindhout DA, Higbee J, et al. Non-homeostatic body weight regulation through a brainstem-restricted receptor for GDF15. Nature. 2017; 550:255–259.
Article35. Mullican SE, Lin-Schmidt X, Chin CN, Chavez JA, Furman JL, Armstrong AA, et al. GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat Med. 2017; 23:1150–1157.
Article36. Yang L, Chang CC, Sun Z, Madsen D, Zhu H, Padkjær SB, et al. GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand. Nat Med. 2017; 23:1158–1166.
Article37. D'Cunha J, Knight E Jr, Haas AL, Truitt RL, Borden EC. Immunoregulatory properties of ISG15, an interferon-induced cytokine. Proc Natl Acad Sci U S A. 1996; 93:211–215.38. Speer SD, Li Z, Buta S, Payelle-Brogard B, Qian L, Vigant F, et al. ISG15 deficiency and increased viral resistance in humans but not mice. Nat Commun. 2016; 7:11496.
Article39. Dos Santos PF, Van Weyenbergh J, Delgobo M, Oliveira Patricio D, Ferguson BJ, Guabiraba R, et al. ISG15-induced IL-10 is a novel anti-inflammatory myeloid axis disrupted during active tuberculosis. J Immunol. 2018; 200:1434–1442.
Article40. Jeon YJ, Yoo HM, Chung CH. ISG15 and immune diseases. Biochim Biophys Acta. 2010; 1802:485–496.
Article41. Malakhova OA, Zhang DE. ISG15 inhibits Nedd4 ubiquitin E3 activity and enhances the innate antiviral response. J Biol Chem. 2008; 283:8783–8787.
Article42. González-Sanz R, Mata M, Bermejo-Martín J, álvarez A, Cortijo J, Melero JA, et al. ISG15 is upregulated in respiratory syncytial virus infection and reduces virus growth through protein ISGylation. J Virol. 2016; 90:3428–3438.
Article43. Zhang X, Bogunovic D, Payelle-Brogard B, Francois-Newton V, Speer SD, Yuan C, et al. Human intracellular ISG15 prevents interferon-α/β over-amplification and auto-inflammation. Nature. 2015; 517:89–93.
Article44. Shapiro SD, Kobayashi DK, Ley TJ. Cloning and characterization of a unique elastolytic metalloproteinase produced by human alveolar macrophages. J Biol Chem. 1993; 268:23824–23829.
Article45. Liu SL, Bae YH, Yu C, Monslow J, Hawthorne EA, Castagnino P, et al. Matrix metalloproteinase-12 is an essential mediator of acute and chronic arterial stiffening. Sci Rep. 2015; 5:17189.
Article46. Molet S, Belleguic C, Lena H, Germain N, Bertrand CP, Shapiro SD, et al. Increase in macrophage elastase (MMP-12) in lungs from patients with chronic obstructive pulmonary disease. Inflamm Res. 2005; 54:31–36.
Article47. Niu H, Li Y, Li H, Chi Y, Zhuang M, Zhang T, et al. Matrix metalloproteinase 12 modulates high-fat-diet induced glomerular fibrogenesis and inflammation in a mouse model of obesity. Sci Rep. 2016; 6:20171.
Article48. Nénan S, Boichot E, Lagente V, Bertrand CP. Macrophage elastase (MMP-12): a pro-inflammatory mediator? Mem Inst Oswaldo Cruz. 2005; 100:Suppl 1. 167–172.
Article49. Wang X, Liang J, Koike T, Sun H, Ichikawa T, Kitajima S, et al. Overexpression of human matrix metalloproteinase-12 enhances the development of inflammatory arthritis in transgenic rabbits. Am J Pathol. 2004; 165:1375–1383.
Article50. Marchant DJ, Bellac CL, Moraes TJ, Wadsworth SJ, Dufour A, Butler GS, et al. A new transcriptional role for matrix metalloproteinase-12 in antiviral immunity. Nat Med. 2014; 20:493–502.
Article51. Zittermann SI, Issekutz AC. Basic fibroblast growth factor (bFGF, FGF-2) potentiates leukocyte recruitment to inflammation by enhancing endothelial adhesion molecule expression. Am J Pathol. 2006; 168:835–846.
Article52. Kanazawa S, Tsunoda T, Onuma E, Majima T, Kagiyama M, Kikuchi K. VEGF, basic-FGF, and TGF-beta in Crohn’s disease and ulcerative colitis: a novel mechanism of chronic intestinal inflammation. Am J Gastroenterol. 2001; 96:822–828.
Article53. Shao X, Chen S, Yang D, Cao M, Yao Y, Wu Z, et al. FGF2 cooperates with IL-17 to promote autoimmune inflammation. Sci Rep. 2017; 7:7024.
Article54. Arese M, Chen Y, Florkiewicz RZ, Gualandris A, Shen B, Rifkin DB. Nuclear activities of basic fibroblast growth factor: potentiation of low-serum growth mediated by natural or chimeric nuclear localization signals. Mol Biol Cell. 1999; 10:1429–1444.
Article55. Ader I, Muller C, Bonnet J, Favre G, Cohen-Jonathan E, Salles B, et al. The radioprotective effect of the 24 kDa FGF-2 isoform in HeLa cells is related to an increased expression and activity of the DNA dependent protein kinase (DNA-PK) catalytic subunit. Oncogene. 2002; 21:6471–6479.
Article56. Li S, Payne S, Wang F, Claus P, Su Z, Groth J, et al. Nuclear basic fibroblast growth factor regulates triple-negative breast cancer chemo-resistance. Breast Cancer Res. 2015; 17:91.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Secreted Proteins of Helicobacter pylori Identified using Pulse-Chase Analysis
- Curcumin Induces Apoptosis and Inhibits Metalloproteinase Activity in Renal Cancer Cell Line
- Possible Roles of UL112-113 Proteins in Human Cytomegalovirus DNA Replication
- Basic Understanding of Iron Metabolism
- Chitinase, Chitinase-like Protein and Allergic Inflammation