Korean J Gastroenterol.
2018 Apr;71(4):204-212. 10.4166/kjg.2018.71.4.204.
The Efficacy of Rebamipide or Ecabet Sodium Supplementation for Helicobacter pylori Eradication Therapy Compared with Quadruple (Concomitant) Regimen
- Affiliations
-
- 1Division of Gastroenterology, Department of Internal Medicine, Gachon University Gil Medical Center, Incheon, Korea. junwonchung@daum.net
- KMID: 2409889
- DOI: http://doi.org/10.4166/kjg.2018.71.4.204
Abstract
- BACKGROUND/AIMS
Although some previous studies reported that a treatment combined with mucoprotective agent could improve the eradication rate in dual or triple therapy, there are other reports that question the efficacy of combining these drugs in concomitant therapy (CoCTx). The aim of this study was to investigate the effects of rebamipide or ecabet on the Helicobacter pylori (H. pylori) eradication combined with CoCTx.
METHODS
We retrospectively reviewed the medical records of 277 patients with proven H. pylori infection. They were assigned to one of 3 regimens for 10 days, twice daily: (a) CoCTx (n=118): lansoprazole 30 mg, amoxicillin 1 g, metronidazole 500 mg, and clarithromycin 500 mg; (b) CoCTx+rebamipide (100 mg) (n=85); (c) CoCTx+ecabet (1 g) (n=74).
RESULTS
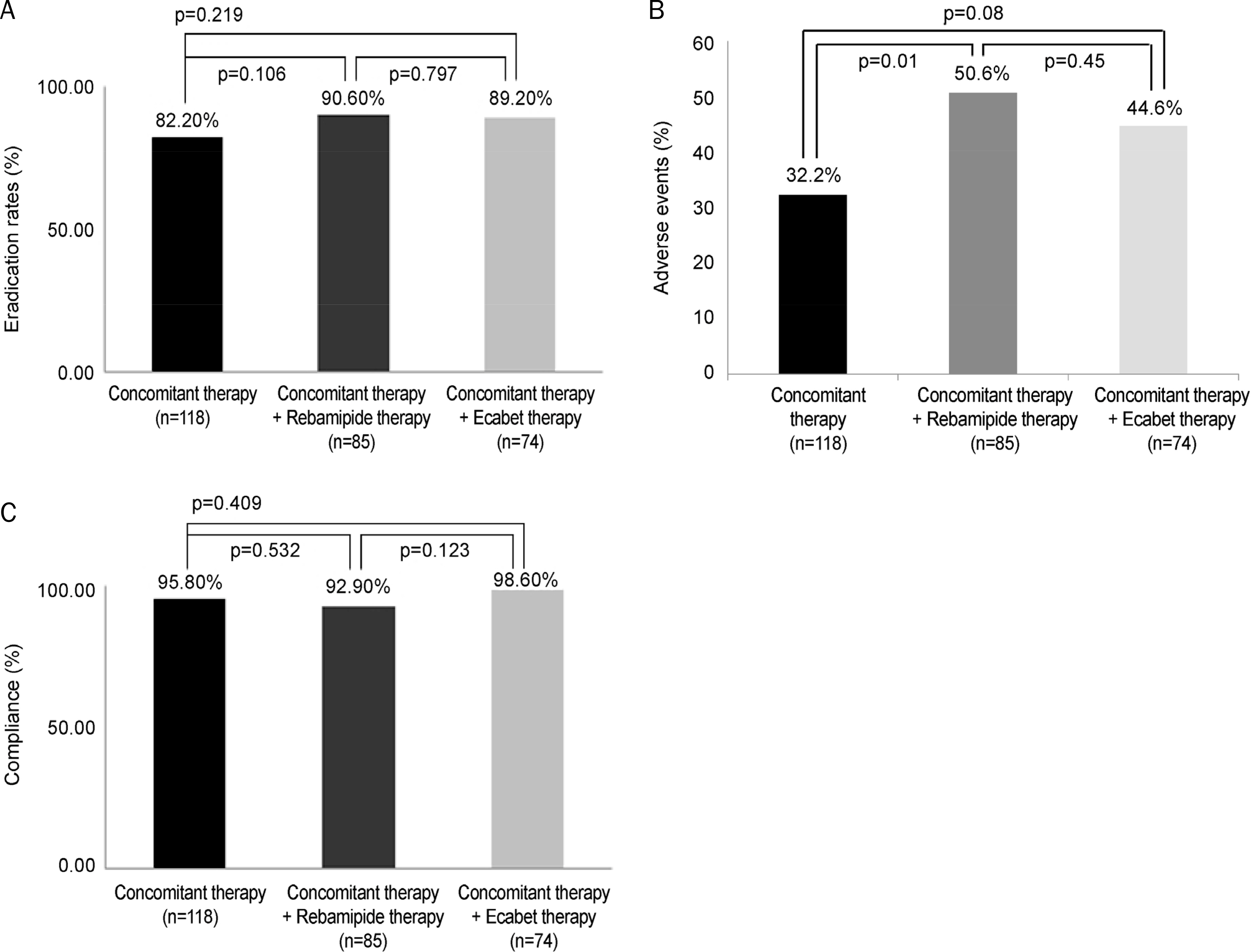
The baseline characteristics were not significantly different. H. pylori eradication rates were 82.2% (97/118) in CoCTx, 90.6% (77/85) in CoCTx+rebamipide, and 89.2% (66/74) in CoCTx+ecabet (p=0.17), which were statistically insignificant. Overall adverse events were more frequently reported in the CoCTx+rebamipide (50.6%. 43/85) and CoCTx+ecabet (44.6%, 33/74) groups than in the CoCTx (32.2%, 38/118) (p = 0.03) group. Drug compliances were not different between three groups (CoCTx: 95.8%, 113/118; CoCT+rebamipide: 92.9%, 79/85; CoCTx+ecabet 98.6%,73/74) (p=0.209). Multivariate analysis showed that the risk of eradication failure was significantly increased with decreased drug compliance (odds ratio 3.52, 95% confidence interval 1.00-12.32; p=0.05).
CONCLUSIONS
Addition of these mucoprotective agent was not superior to CoCTx alone for eradicating H. pylori infection with frequent adverse events. Rather, drug compliance is the most related factor affecting the eradication rate. Our data suggest the importance of drug compliance over the drugs used.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Hunt RH, Xiao SD, Megraud F, et al. Helicobacter pylori in developing countries. World Gastroenterology Organisation Global Guideline. J Gastrointestin Liver Dis. 2011; 20:299–304.2. McColl KE. Clinical practice. Helicobacter pylori infection. N Engl J Med. 2010; 362:1597–1604.3. Kim N, Kim JJ, Choe YH, et al. Diagnosis and treatment guidelines for Helicobacter pylori infection in Korea. Korean J Gastroenterol. 2009; 54:269–278.
Article4. Katelaris PH, Forbes GM, Talley NJ, Crotty B. A randomized comparison of quadruple and triple therapies for Helicobacter pylori eradication: the QUADRATE Study. Gastroenterology. 2002; 123:1763–1769.
Article5. Kim SG, Jung HK, Lee HL, et al. Guidelines for the diagnosis and treatment of Helicobacter pylori infection in Korea, 2013 revised edition. Korean J Gastroenterol. 2013; 62:3–26.
Article6. Lee JW, Kim N, Kim JM, et al. Prevalence of primary and secondary antimicrobial resistance of Helicobacter pylori in Korea from 2003 through 2012. Helicobacter. 2013; 18:206–214.7. Chuang CH, Sheu BS, Kao AW, et al. Adjuvant effect of vitamin C on omeprazole-amoxicillin-clarithromycin triple therapy for Helicobacter pylori eradication. Hepatogastroenterology. 2007; 54:320–324.8. de Bortoli N, Leonardi G, Ciancia E, et al. Helicobacter pylori eradication: a randomized prospective study of triple therapy versus triple therapy plus lactoferrin and probiotics. Am J Gastroenterol. 2007; 102:951–956.
Article9. Kim JM, Kim JS, Jung HC, et al. The effects of ecabet sodium on nuclear factor-kappa B activation and chemokine gene expression in Helicobacter pylori-infected human gastric epithelial cells. Korean J Med. 2003; 65:178–187.10. Shimoyama T, Fukuda S, Liu Q, Nakaji S, Munakata A, Sugawara K. Ecabet sodium inhibits the ability of Helicobacter pylori to induce neutrophil production of reactive oxygen species and inter-leukin-8. J Gastroenterol. 2001; 36:153–157.
Article11. Shibata K, Kasuga O, Yasoshima A, Matsushita T, Kawakami Y. Bactericidal effect of ecabet sodium on clarithromycin- and metronidazole-resistant clinical isolates of Helicobacter pylori. Jpn J Antibiot. 1997; 50:525–531.12. Arakawa T, Watanabe T, Fukuda T, Yamasaki K, Kobayashi K. Rebamipide, novel prostaglandin-inducer, accelerates healing and reduces relapse of acetic acid-induced rat gastric ulcer. Comparison with cimetidine. Dig Dis Sci. 1995; 40:2469–2472.
Article13. Hahm KB, Lee KJ, Kim YS, et al. Quantitative and qualitative usefulness of rebamipide in eradication regimen of Helicobacter pylori. Dig Dis Sci. 1998; 43(9 Suppl):192S–197S.14. Suzuki M, Miura S, Mori M, et al. Rebamipide, a novel antiulcer agents, attenuates Helicobacter pylori induced gastric mucosal cell injury associated with neutrophil derived oxidants. Gut. 1994; 35:1375–1378.15. Murakami K, Okajima K, Uchiba M, et al. Rebamipide attenuates indomethacin-induced gastric mucosal lesion formation by inhibiting activation of leukocytes in rats. Dig Dis Sci. 1997; 42:319–325.16. Hayashi S, Sugiyama T, Asaka M, Yokota K, Oguma K, Hirai Y. Modification of Helicobacter pylori adhesion to human gastric epithelial cells by antiadhesion agents. Dig Dis Sci. 1998; 43(9 Suppl):56S–60S.17. Lee HJ, Kim JI, Lee JS, et al. Concomitant therapy achieved the best eradication rate for Helicobacter pylori among various treatment strategies. World J Gastroenterol. 2015; 21:351–359.18. Kim SY, Lee SW, Choe JW, et al. Helicobacter pylori eradication rates of concomitant and sequential therapies in Korea. Helicobacter. 2017; 22:e12441.
Article19. Chung JW, Han JP, Kim KO, et al. Ten-day empirical sequential or concomitant therapy is more effective than triple therapy for Helicobacter pylori eradication: a multicenter, prospective study. Dig Liver Dis. 2016; 48:888–892.
Article20. Lee DS, Ahn BM, Lee KM, et al. Effect of rebamipide (Mucosta(R)) in eradication of Helicobacter pylori. Korean J Gastrointest Endosc. 2000; 21:832–837.21. Shimoyama T, Fukuda Y, Fukuda S, Munakata A, Yoshida Y, Shimoyama T. Ecabet sodium eradicates Helicobacter pylori infection in gastric ulcer patients. J Gastroenterol. 1996; 31(Suppl 9):59–62.22. Kagaya H, Kato M, Komatsu Y, et al. High-dose ecabet sodium improves the eradication rate of Helicobacter pylori in dual therapy with lansoprazole and amoxicillin. Aliment Pharmacol Ther. 2000; 14:1523–1527.
Article23. Seo JY, Kim MJ, Ko KH, Kim DH, Lim DS, Chon HR. Efficacy of ecabet sodium for Helicobacter pylori eradication, combined with lansoprazole-based triple regimen: a prospective study. Korean J Med. 2011; 80:546–552.24. Kim JY, Lee DH, Son JH, et al. Effect of additional ecabet sodium on conventional triple therapy for Helicobacter pylori eradication in Korea. Korean J Gastrointest Endosc. 2011; 42:349–355.25. Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010; 59:1143–1153.
Article26. Kim JM. Antibiotic resistance of Helicobacter pylori isolated from Korean patients. Korean J Gastroenterol. 2006; 47:337–349.27. Kim N, Kim JM, Kim CH, et al. Institutional difference of antibiotic resistance of Helicobacter pylori strains in Korea. J Clin Gastroenterol. 2006; 40:683–687.
Article28. Kim BK, Kim JS, Kim BW, Ji JS, Choi H, Park SM. Annual eradication rates of Helicobacter pylori infection over 9 years in Incheon. Korean J Helicobacter Up Gastrointest Res. 2015; 15:103–107.29. Graham DY, Lee YC, Wu MS. Rational Helicobacter pylori therapy: evidencebased medicine rather than medicine-based evidence. Clin Gastroenterol Hepatol. 2014; 12:177–186. e3;Discussion e12-e13.
Article30. Wu JY, Liou JM, Graham DY. Evidence-based recommendations for successful Helicobacter pylori treatment. Expert Rev Gastroenterol Hepatol. 2014; 8:21–28.31. Chung JW, Jung YK, Kim YJ, et al. Ten-day sequential versus triple therapy for Helicobacter pylori eradication: a prospective, openlabel, randomized trial. J Gastroenterol Hepatol. 2012; 27:1675–1680.32. Nishizawa T, Nishizawa Y, Yahagi N, Kanai T, Takahashi M, Suzuki H. Effect of supplementation with rebamipide for Helicobacter pylori eradication therapy: a systematic review and metaanalysis. J Gastroenterol Hepatol. 2014; 29(Suppl 4):20–24.
Article33. Wang Y, Wang B, Lv ZF, et al. Efficacy and safety of ecabet sodium as an adjuvant therapy for Helicobacter pylori eradication: a systematic review and metaanalysis. Helicobacter. 2014; 19:372–381.34. Kagaya H, Kato M, Kobayashi T, et al. Study on the effect of a new anti-ulcer agent, ecabet sodium, on the dual therapy for eradication of Helicobacter pylori in randomized patients. Gastroenterololy. 1998; 114:A164.
Article35. Kim HW, Kim GH, Cheong JY, et al. H pylori eradication: a randomized prospective study of triple therapy with or without ecabet sodium. World J Gastroenterol. 2008; 14:908–912.
Article36. Ohkusa T, Takashimizu I, Fujiki K, et al. Prospective evaluation of a new anti-ulcer agent, ecabet sodium, for the treatment of Helicobacter pylori infection. Aliment Pharmacol Ther. 1998; 12:457–461.
Article37. Zullo A, De Francesco V, Hassan C, Morini S, Vaira D. The sequential therapy regimen for Helicobacter pylori eradication: a pooled-data analysis. Gut. 2007; 56:1353–1357.
Article38. Georgopoulos SD, Xirouchakis E, Martinez-Gonzalez B, et al. Clinical evaluation of a ten-day regimen with esomeprazole, metronidazole, amoxicillin, and clarithromycin for the eradication of Helicobacter pylori in a high clarithromycin resistance area. Helicobacter. 2013; 18:459–467.39. Chung JW, Lee JH, Jung HY, et al. Second-line Helicobacter pylori eradication: a randomized comparison of 1-week or 2-week bismuth-containing quadruple therapy. Helicobacter. 2011; 16:289–294.
Article40. Kim N, Lim CN, Lim SH, et al. Establishment of an Helicobacter pylori-eradication regimen in consideration of drug resistance, recrudescence and reinfection rate of H. pylori. Korean J Med. 1999; 56:279–291.41. Murakami K, Sato R, Okimoto T, et al. Efficacy of triple therapy comprising rabeprazole, amoxicillin and metronidazole for second-line Helicobacter pylori eradication in Japan, and the influence of metronidazole resistance. Aliment Pharmacol Ther. 2003; 17:119–123.
Article42. Kim JM, Kim JS, Kim N, Jung HC, Song IS. Distribution of fluoroquinolone MICs in Helicobacter pylori strains from Korean patients. J Antimicrob Chemother. 2005; 56:965–967.
Article43. Kim JM, Kim JS, Jung HC, Kim N, Kim YJ, Song IS. Distribution of antibiotic MICs for Helicobacter pylori strains over a 16-year period in patients from Seoul, South Korea. Antimicrob Agents Chemother. 2004; 48:4843–4847.44. Suzuki S, Suzuki H, Nishizawa T, et al. Past rifampicin dosing determines rifabutin resistance of Helicobacter pylori. Digestion. 2009; 79:1–4.45. Nam TM, Lee DH, Kang KP, et al. Clinical factors that potentially affect the treatment outcome of Helicobacter pylori eradication therapy with using a standard triple regimen in peptic ulcer patients. Korean J Gastrointest Endosc. 2008; 36:200–205.46. Suzuki T, Matsuo K, Ito H, et al. Smoking increases the treatment failure for Helicobacter pylori eradication. Am J Med. 2006; 119:217–224.
Article47. Brenner H, Rothenbacher D, Bode G, Adler G. Relation of smoking and alcohol and coffee consumption to active Helicobacter pylori infection: cross sectional study. BMJ. 1997; 315:1489–1492.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy of Ecabet Sodium for Helicobacter pylori Eradication, Combined with Lansoprazole-Based Triple Regimen: A Prospective Study
- Comparison of the Efficacy of 12-day Concomitant Quadruple Therapy versus 14-day High dose Dual Therapy as a First-line H. pylori Eradication Regimen
- Effect of Additional Ecabet Sodium on Conventional Triple Therapy for Helicobacter pylori Eradication in Korea
- Effect of Ecabet Sodium as an Adjuvant Therapy for Helicobacter pylori Eradication with Sequential Therapy
- Effect of Omeprazole Quadruple Therapyon Eradication of Helicobacter pylori