Ann Lab Med.
2018 Jul;38(4):338-347. 10.3343/alm.2018.38.4.338.
Allergen Microarrays for In Vitro Diagnostics of Allergies: Comparison with ImmunoCAP and AdvanSure
- Affiliations
-
- 1Department of Biomedical Engineering (BK21 Plus), Dongguk University, Seoul, Korea. ykwon@dongguk.edu
- 2Department of Otolaryngology, Gil Medical Center, Gachon University, Incheon, Korea. kst2383@gilhospital.com
- 3Department of Research and Development, Won Medical Co., Bucheon, Korea.
- KMID: 2408073
- DOI: http://doi.org/10.3343/alm.2018.38.4.338
Abstract
- BACKGROUND
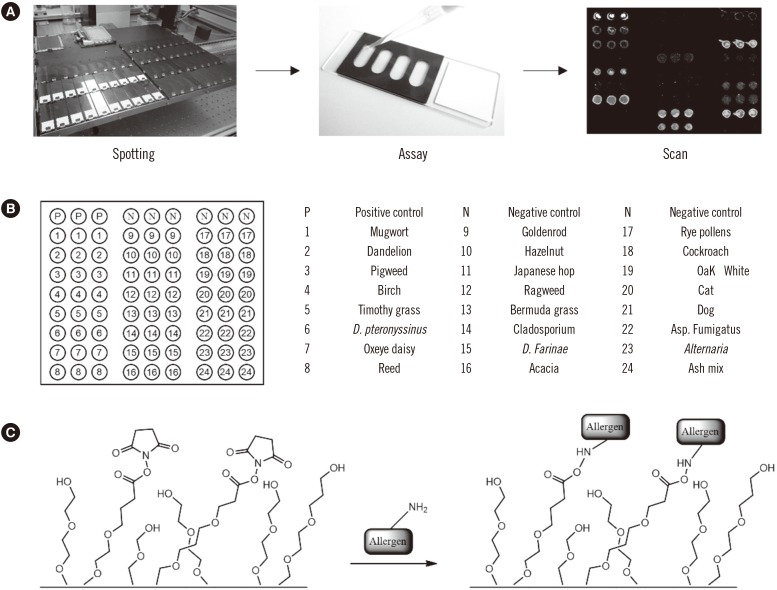
In vitro detection of the allergen-specific IgE antibody (sIgE) is a useful tool for the diagnosis and treatment of allergies. Although multiple simultaneous allergen tests offer simple and low-cost screening methods, these platforms also have limitations with respect to multiplexibility and analytical performance. As an alternative assay platform, we developed and validated a microarray using allergen extracts that we termed "GOLD" chip.
METHODS
Serum samples of 150 allergic rhinitis patients were used in the study, and the diagnostic performance of the microarray was compared with that of AdvanSure (LG Life Sciences, Daejun, Korea) and ImmunoCAP (Phadia, Uppsala, Sweden). Standard IgE samples were used for the quantitative measurement of sIgEs.
RESULTS
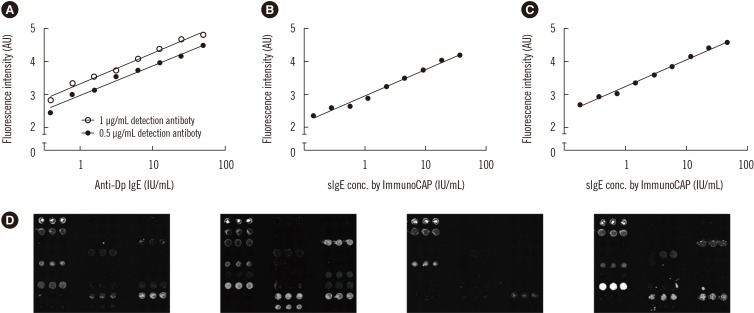
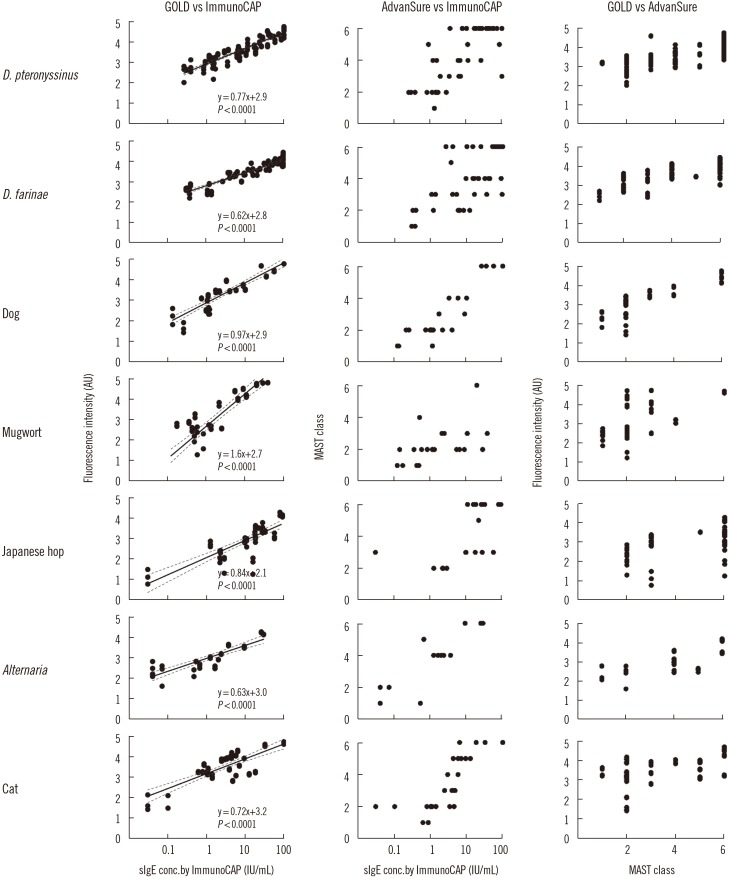
The microarray-based assay showed excellent performance in the quantitative measurement of sIgEs, demonstrating a linear correlation within the range of sIgE concentrations tested. The limit of detection (LOD) was lower than 0.35 IU/mL, which is the current standard for the LOD cut-off. The assay also provided highly reproducible sets of data. The total agreement percentage of positive and negative calls was 92.2% compared with ImmunoCAP. Moreover, an outstanding correlation was observed between the microarray and the ImmunoCAP results, with Cohen's kappa and Pearson correlation coefficient values of 0.80 and 0.79, respectively.
CONCLUSIONS
The microarray-based in vitro diagnostic platform offers a sensitive, reproducible, and highly quantitative method to detect sIgEs. The results showed strong correlations with that of ImmunoCAP. These results suggest that the new allergen microarray can serve as a useful alternative to current screening platforms, ultimately becoming a first-line screening method.
Keyword
MeSH Terms
Figure
Reference
-
1. Heinzerling L, Mari A, Bergmann KC, Bresciani M, Burbach G, Darsow U, et al. The skin prick test - European standards. Clin Transl Allergy. 2013; 3:3. PMID: 23369181.2. Liccardi G, D'Amato G, Canonica GW, Salzillo A, Piccolo A, Passalacqua G. Systemic reactions from skin testing: literature review. J Investig Allergol Clin Immunol. 2006; 16:75–78.3. Ownby DR. Allergy testing: in vivo versus in vitro. Pediatr Clin North Am. 1988; 35:995–1009. PMID: 3050840.4. Berg TLO, Johansson SGO. Allergy diagnosis with the radioallergosorbent test. J Allergy Clin Immunol. 1974; 54:209–221. PMID: 4411916.5. Gleeson M, Cripps AW, Hensley MJ, Wlodarczyk JH, Henry RL, Clancy RL. A clinical evaluation in children of the Pharmacia ImmunoCAP system for inhalant allergens. Clin Exp Allergy. 1996; 26:697–702. PMID: 8809427.6. Kelso JM, Sodhi N, Gosselin VA, Yunginger JW. Diagnostic performance characteristics of the standard Phadebas RAST, modified RAST, and Pharmacia CAP system versus skin testing. Ann Allergy. 1991; 67:511–514. PMID: 1958005.7. Park KH, Lee J, Sim DW, Lee SC. Comparison of singleplex specific IgE detection immunoassays: ImmunoCAP Phadia 250 and Immulite 2000 3gAllergy. Ann Lab Med. 2018; 38:23–31. PMID: 29071815.8. Banker DD, Daftary VG, Daftary GV, Bhandari NM, Dayanand SR. Allergy diagnosis by Pharmacia Cap System. Indian J Med Sci. 1999; 53:387–389. PMID: 10710831.9. Han MK, Seo MH, Lee D, Kim SH, Park HS, Kim HS. Optimization of critical factors affecting the performance of an allergen chip for the analysis of an allergen-specific human IgE in serum. Anal Sci. 2007; 23:545–549. PMID: 17495399.10. Melioli G, Marcomini L, Agazzi A, Bazurro G, Tosca M, Rossi GA, et al. The IgE repertoire in children and adolescents resolved at component level: a cross-sectional study. Pediatr Allergy Immunol. 2012; 23:433–440. PMID: 22103266.11. Rim JH, Park BG, Kim JH, Kim HS. Comparison and clinical utility evaluation of four multiple allergen simultaneous tests including two newly introduced fully automated analyzers. Pract Lab Med. 2016; 4:50–61. PMID: 28856193.12. Park KH, Lee J, Lee SC, Son YW, Sim DW, Lee JH, et al. Comparison of the ImmunoCAP Assay and AdvanSure™ AlloScreen Advanced Multiplex Specific IgE Detection Assay. Yonsei Med J. 2017; 58:786–792. PMID: 28540992.13. Cho JH, Suh JD, Kim JK, Hong SC, Park IH, Lee HM. Correlation between skin-prick testing, individual specific IgE tests, and a multiallergen IgE assay for allergy detection in patients with chronic rhinitis. Am J Rhinol Allergy. 2014; 28:388–391. PMID: 25198024.14. Templin MF, Stoll D, Schrenk M, Traub PC, Vohringer CF, Joos TO. Protein microarray technology. Trends Biotechnol. 2002; 20:160–166. PMID: 11906748.15. Jeon HJ, Lee MH, Jang WH, Kwon YK. Intein-mediated protein engineering for biosensor fabrication. Biochip J. 2016; 10:277–287.16. Park HJ, Lee JH, Park KH, Ann HW, Jin MN, Choi SY, et al. A nationwide survey of inhalant allergens sensitization and levels of indoor major allergens in Korea. Allergy Asthma Immunol Res. 2014; 6:222–227. PMID: 24843797.17. Choi JS, Roh JY, Lee JR. Clinical availability of component-resolved diagnosis using microarray technology in atopic dermatitis. Ann Dermatol. 2014; 26:437–446. PMID: 25143671.18. Kim J, Hahm MI, Lee SY, Kim WK, Chae Y, Park YM, et al. Sensitization to aeroallergens in Korean children: a population-based study in 2010. J Korean Med Sci. 2011; 26:1165–1172. PMID: 21935271.19. Lebrun SJ, Petchpud WN, Hui A, McLaughlin CS. Development of a sensitive, colorometric microarray assay for allergen-responsive human IgE. J Immunol Methods. 2005; 300:24–31. PMID: 15893759.20. Fall BI, Eberlein-Konig B, Behrendt H, Niessner R, Ring J, Weller MG. Microarrays for the screening of allergen-specific IgE in human serum. Anal Chem. 2003; 75:556–562. PMID: 12585484.21. Kim TE, Park SW, Cho NY, Choi SY, Yong TS, Nahm BH, et al. Quantitative measurement of serum allergen-specific IgE on protein chip. Exp Mol Med. 2002; 34:152–158. PMID: 12085991.22. Wiltshire S, O'Malley S, Lambert J, Kukanskis K, Edgar D, Kingsmore SF, et al. Detection of multiple allergen-specific IgEs on microarrays by immunoassay with rolling circle amplification. Clin Chem. 2000; 46:1990–1993. PMID: 11106333.23. Jahn-Schmid B, Harwanegg C, Hiller R, Bohle B, Ebner C, Scheiner O, et al. Allergen microarray: comparison of microarray using recombinant allergens with conventional diagnostic methods to detect allergen-specific serum immunoglobulin E. Clin Exp Allergy. 2003; 33:1443–1449. PMID: 14519153.24. Ornberg RL, Harper TF, Liu H. Western blot analysis with quantum dot fluorescence technology: a sensitive and quantitative method for multiplexed proteomics. Nat Methods. 2005; 2:79–81.25. Deinhofer K, Sevcik H, Balic N, Harwanegg C, Hiller R, Rumpold H. Microarrayed allergens for IgE profiling. Methods. 2004; 32:249–254. PMID: 14962759.26. Kimiko T, Kunie K, Hitoshi T, Hiroaki M, Satoshi N, Eishin M. Usability of Fag e 2 ImmunoCAP in the diagnosis of buckwheat allergy. Arch Dermatol Res. 2011; 303:635–642. PMID: 21461893.27. Lucassen R, Schulte-Pelkum J, Csuvarszki C, Kleine-Tebbe J, Fooke M, Mahler M. Evaluation of a novel rapid test system for the detection of allergic sensitization to Timothy grass pollen against established laboratory methods and skin prick test. J Allergy (Cairo). 2010; 2010:pii: 524084. DOI: 10.1155/2010/524084.28. Saarinen KM, Suomalainen H, Savilahti E. Diagnostic value of skin-prick and patch tests and serum eosinophil cationic protein and cow's milkspecific IgE in infants with cow's milk allergy. Clin Exp Allergy. 2001; 31:423–429. PMID: 11260154.29. Grier TJ. Laboratory methods for allergen extract analysis and quality control. Clin Rev Allergy Immunol. 2001; 21:111–140. PMID: 11725601.30. Andri L, Senna G, Betteli C, Givanni S, Andri G, Dimitri G, et al. Local nasal immunotherapy with extract in powder form is effective and safe in grass pollen rhinitis: a double-blind study. J Allergy Clin Immunol. 1996; 97:34–41. PMID: 8568135.31. Wagenlehner FM, Bschleipfer T, Pilatz A, Weidner W. Pollen extract for chronic prostatitis-chronic pelvic pain syndrome. Urol Clin North Am. 2011; 38:285–292. PMID: 21798390.32. Jung JH, Kang IG, Kim ST. Comparison of component-resolved diagnosis by using allergen microarray with the conventional tests in allergic rhinitis patients: the first using in Korea. Clin Exp Otorhinolaryngol. 2015; 8:385–389. PMID: 26622959.33. Schuurman J, Perdok GJ, Lourens TE, Parren PW, Chapman MD, Aalberse RC. Production of a mouse/human chimeric IgE monoclonal antibody to the house dust mite allergen Der p 2 and its use for the absolute quantification of allergen-specific IgE. J Allergy Clin Immunol. 1997; 99:545–550. PMID: 9111501.34. Lupinek C, Wollmann E, Baar A, Banerjee S, Breiteneder H, Broecker BM. Advances in allergen-microarray technology for diagnosis and monitoring of allergy: the MeDALL allergen-chip. Methods. 2014; 66:106–119. PMID: 24161540.35. Curin M, Swoboda I, Wollmann E, Lupinek C, Spitzauer S, van Hage M, et al. Microarrayed dog, cat, and horse allergens show weak correlation between allergen-specific IgE and IgG responses. J Allergy Clin Immunol. 2014; 133:918–921. PMID: 24406070.36. Pomponi D, Di Zenzo G, Zennaro D, Calabresi V, Eming R, Zuzzi S, et al. Detection of IgG and IgE reactivity to BP180 using the ISAC® microarray system. Br J Dermatol. 2013; 168:1205–1214. PMID: 23252883.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Component-Resolved Diagnosis by Using Allergen Microarray With the Conventional Tests in Allergic Rhinitis Patients: The First Using in Korea
- Detection of Allergen Specific IgE by AdvanSure Allergy Screen Test
- Performance Comparison of ImmunoCAP and HYTEC 288 in the Quantitative Tests of Allergen-specific IgE
- A case of a 6-year-old boy diagnosed with lipid transfer protein syndrome using the ImmunoCAP and ImmunoCAP Immuno Solid-phase Allergen Chip
- A Case of an Infant Diagnosed with Cow's Milk Allergy and Concurrent Meat Allergy via ImmunoCAP ISAC®