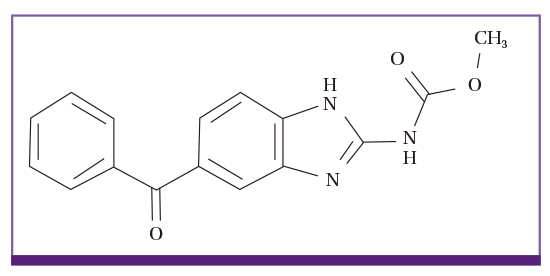
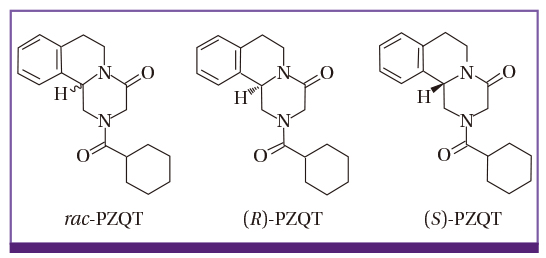
Infect Chemother.
2018 Mar;50(1):1-10. 10.3947/ic.2018.50.1.1.
Albendazole and Praziquantel: Review and Safety Monitoring in Korea
- Affiliations
-
- 1Department of Parasitology and Tropical Medicine, Seoul National University College of Medicine, Seoul, Korea. hst@snu.ac.kr
- KMID: 2407962
- DOI: http://doi.org/10.3947/ic.2018.50.1.1
Abstract
- Albendazole (ADZ) and praziquantel (PZQT) have been used as anthelmintics for over 30 years. Worldwide, hundreds of millions tablets are administered to people and livestock every year. ADZ is poorly orally absorbed (< 5%), and its uptake is enhanced by high-fat meals, while PZQT is well absorbed (> 75%) and uptake is enhanced by carbohydrate-rich meals. Both ADZ and PZQT are safe, but not recommended for children < 2 years or for women in the first trimester of pregnancy. Serious adverse events occur following high dose and prolonged administration of these drugs for treatment of echinococcosis or neurocysticercosis, especially in patients with poor liver function. The adverse events may be induced by the drugs, or by the dead worms themselves. The Korea Institute of Drug Safety & Risk Management monitors drug-related adverse events in Korea, and its database included 256 probable or possible ADZ-associated events and 108 PZQT-associated events between 2006 and 2015. Such low incidence rates in Korea are due to the low single dose treatments of ADZ, and the short-term use of PZQT. The number of serious adverse events due to drug interaction induced by ADZ and PZQT were six and two, respectively. We conclude that ADZ and PZQT are generally safe drugs, but they must be used with caution in people with poor liver function or those being comedicated for gastroesophageal reflux disease.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Status of common parasitic diseases in Korea in 2019
Sun Huh
J Korean Med Assoc. 2019;62(8):437-456. doi: 10.5124/jkma.2019.62.8.437.
Reference
-
1. Albonico M, Levecke B, LoVerde PT, Montresor A, Prichard R, Vercruysse J, Webster JP. Monitoring the efficacy of drugs for neglected tropical diseases controlled by preventive chemotherapy. J Glob Antimicrob Resist. 2015; 3:229–236.
Article2. World Health Organization (WHO). Update on the global status of the donation managed by WHO of the medicines for preventive chemotherapy (PC) 14 November 2017. Accessed 28 December 2017. Available at: http://www.who.int/neglected_diseases/preventive_chemotherapy/PC_medicines.pdf?ua=1.3. Verrest L, Dorlo TP. Lack of clinical pharmacokinetic studies to optimize the treatment of neglected tropical diseases: a systematic review. Clin Pharmacokinet. 2017; 56:583–606.
Article4. Dayan AD. Albendazole, mebendazole and praziquatnel, Review of non-clinical toxicity and pharmacokinetics. Acta Trop. 2003; 86:141–159.
Article5. Nagy J, Schipper HG, Koopmans RP, Butter JJ, Van Boxtal CJ, Kager PA. Effect of grapefruit juice or cimetidine coadministration on albendazole bioavailability. Am J Trop Med Hyg. 2002; 66:260–263.
Article6. Pion SD, Chesnais CB, Bopda J, Louya F, Fischer PU, Majewski AC, Weil GJ, Boussinesq M, Missamou F. The impact of two semiannual treatments with albendazole alone on lymphatic filariasis and soil-transmitted helminth infections: a community-based study in the Republic of Congo. Am J Trop Med Hyg. 2015; 92:959–966.
Article7. Moser W, Coulibaly JT, Ali SM, Ame SM, Amour AK, Yapi RB, Albonico M, Puchkov M, Huwyler J, Hattendorf J, Keiser J. Efficacy and safety of tribendimidine, tribendimidine plus ivermectin, tribendimidine plus oxantel pamoate, and albendazole plus oxantel pamoate against hookworm and concomitant soil-transmitted helminth infections in Tanzania and Côte d’Ivoire: a randomised, controlled, single-blinded, non-inferiority trial. Lancet Infect Dis. 2017; 17:1162–1171.
Article8. Thomsen EK, Sanuku N, Baea M, Satofan S, Maki E, Lombore B, Schmidt MS, Siba PM, Weil GJ, Kazura JW, Fleckenstein LL, King CL. Efficacy, safety, and pharmacokinetics of coadministered diethylcarbamazine, albendazole, and ivermectin for treatment of bancroftian filariasis. Clin Infect Dis. 2016; 62:334–341.
Article9. Moroni S, Moscatelli G, Bournissen FG, González N, Ballering G, Freilij H, Salgueiro F, Altcheh J. Abdominal cystic echinococcosis treated with albendazole. A Pediatric Cohort Study. PLoS One. 2016; 11:e0160472.
Article10. Mohapatra S, Sahoo AJ. Drug-induced psychosis associated with albendazole-ivermectin combination therapy in a 10-year-old child. J Cjild Adolesc Psychopjarmacol. 2015; 25:817–818.
Article11. Hogan J, Dehoux L, Niel O, Elenga N, Deschênes G, Dauger S. Hemolytic anemia and irreversible kidney and brain injuries after accidental intravenous injection of albendazole suspension in an infant. Clin Toxicol. 2016; 54:72–73.
Article12. Tas A, Köklü S, Celik H. Loss of body hair as a side effect of albendazole. Wien Klin Wochenschr. 2012; 124:220.
Article13. Choi GY, Yang HW, Cho SH, Kang DW, Go H, Lee WC, Lee YJ, Jung SH, Kim AN, Cha SW. Acute drug-induced hepatitis caused by albendazole. J Korean Med Sci. 2008; 23:903–905.
Article14. Marin Zuluaga JI, Marin Castro AE, Perez Cadavid JC, Restrepo Gutierrez JC. Albendazole-induced granulomatous hepatitis: a case report. J Med Case Reports. 2013; 7:201.
Article15. Amoruso C, Fuoti M, Miceli V, Zito E, Celano MR, De Giorgi A, Nebbia G. Acute hepatitis as a side effect of albendazole: a pediatric case. Pediatr Med Chir. 2009; 31:262–264.16. Opatrny L, Prichard R, Snell L, Maclean JD. Death related to albendazole-induced pancytopenia: case report and review. Am J Trop Med Hyg. 2005; 72:291–294.
Article17. Borgsteede FH, Dercksen DD, Huijbers R. Doramectin and albendazole resistance in sheep in The Netherlands. Vet Parasitol. 2007; 144:180–183.
Article18. Rashwan N, Bourguinat C, Keller K, Gunawardena NK, de Silva N, Prichard R. Isothermal diagnostic assays for monitoring single nucleotide polymorphisms in Necator americanus associated with benzimidazole drug resistance. PLoS Negl Trop Dis. 2016; 10:e0005113.19. Jaeger LH, Carvalho-Costa FA. Status of benzimidazole resistance in intestinal nematode populations of livestock in Brazil: a systematic review. BMC Vet Res. 2017; 13:358.
Article20. Castro LS, Kviecinski MR, Ourique F, Parisotto EB, Grinevicius VM, Correia JF, Wilhelm Filho D, Pedrosa RC. Albendazole as a promising molecule for tumor control. Redox Biol. 2016; 10:90–99.
Article21. Kang BS, Choi JS, Lee SE, Lee JK, Kim TH, Jang WS, Tunsirikongkon A, Kim JK, Park JS. Enhancing the in vitro anticancer activity of albendazole incorporated into chitosan-coated PLGA nanoparticles. Carbohydr Polym. 2017; 159:39–47.
Article22. Movahedi F, Li L, Gu W, Xu ZP. Nanoformulations of albendazole as effective anticancer and antiparasite agents. Nanomedicine (Lond). 2017; 12:2555–2574.
Article23. Choi JS, Han JY, Ahn HK, Ryu HM, Koren G. Foetal outcomes after exposure to albendazole in early pregnancy. J Obstet Gynaecol. 2017; 37:1108–1111.
Article24. Salam RA, Haider BA, Humayun Q, Bhutta ZA. Effect of administration of antihelminthics for soil-transmitted helminths during pregnancy. Cochrane Database Syst Rev. 2015; CD005547.
Article25. Sun Q, Mao R, Wang D, Hu C, Zheng Y, Sun D. The cytotoxicity study of praziquantel enantiomers. Drug Des Devel Ther. 2016; 10:2061–2068.
Article26. Chai JY. Prqaziquantel treatment in trematode and cestode infections: an update. Infect Chemother. 2013; 45:32–43.
Article27. Kovač J, Vargas M, Keiser J. In vitro and in vivo activity of R- and S- praziquantel enantiomers and the main human metabolite trans-4-hydroxy-praziquantel against Schistosoma haematobium . Parasit Vectors. 2017; 10:365.
Article28. Rim HJ. The current pathobiology and chemotherapy of clonorchiasis. Korean J Parasitol. 1986; 24:Suppl. 1–141.
Article29. Zwang J, Olliaro P. Efficacy and safety of praziquantel 40 mg/kg in preschool-aged and school-aged children: a meta-analysis. Parasit Vectors. 2017; 10:47.
Article30. Matthaiou DK, Panos G, Adamidi ES, Falagas ME. Albendazole versus praziquantel in the treatment of neurocysticercosis: a meta-analysis of comparative trials. PLoS Negl Trop Dis. 2008; 2:e194.
Article31. Shen C, Choi MH, Bae YM, Yu G, Wang S, Hong ST. A case of anaphylactic reaction to praziquantel treatment. Am J Trop Med Hyg. 2007; 76:603–605.
Article32. Lee JM, Lim HS, Hong ST. Hypersensitive reaction to praziquantel in a clonorchiasis patient. Korean J Parasitol. 2011; 49:273–275.
Article33. Matsumoto J. Adverse effects of praziquantel treatment of Schistosoma japonicum infection: involvement of host anaphylactic reactions induced by parasite antigen release. Int J Parasitol. 2002; 32:461–471.
Article34. Korea Institute of Drug Safety & Risk Management (KIDS). Accessed 7 July 2016. Available at: https://www.drugsafe.or.kr/en/index.do;jsessionid=dXhaQ96oBqZW22vFNJe2WrzXyK1zJWbbU2zuqhFyUkljnYTzMZRDWN7pYmKxgxWV.webint_2_servlet_engine1.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Albendazole Therapy for Multiple Neurocysticercosis
- Albendazole versus. Praziquantel for Therapy for Neurocysticercosis
- Stereotactic Surgery of Neurocysticercosis
- A Case of Relapsed Subarachnoid Racemose Cysticercosis Successfully Treated with Albendazole
- A Case of Cysticercosis Treated with Praziquantel