J Clin Neurol.
2018 Apr;14(2):158-164. 10.3988/jcn.2018.14.2.158.
Glucose Hypometabolism in Hippocampal Subdivisions in Alzheimer's Disease: A Pilot Study Using High-Resolution ¹â¸F-FDG PET and 7.0-T MRI
- Affiliations
-
- 1Neuroscience Research Institute, Gachon University, Incheon, Korea.
- 2Department of Biomedical Engineering, College of Health Science, Gachon University, Incheon, Korea.
- 3Department of Neurology, Gachon University Gil Medical Center, Incheon, Korea. khpark@gachon.ac.kr
- 4Department of Neurosurgery, Gachon University Gil Medical Center, Incheon, Korea. neurokim@gachon.ac.kr
- KMID: 2407941
- DOI: http://doi.org/10.3988/jcn.2018.14.2.158
Abstract
- BACKGROUND AND PURPOSE
Atrophy of the hippocampus is an important clinical diagnostic marker of Alzheimer's disease (AD), and so assessments of hippocampal activity and its subdivisions might provide invaluable information. This study compared the glucose metabolism of hippocampal subdivisions in mild-AD patients and healthy controls.
METHODS
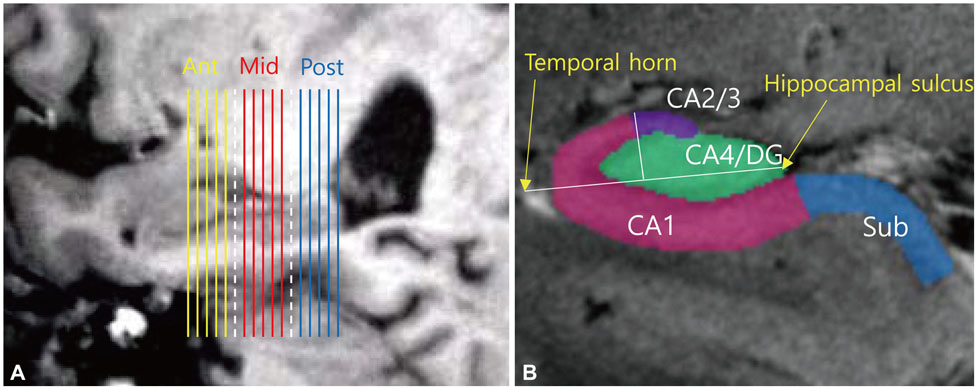
High-resolution T2*-weighted gradient-echo magnetic resonance imaging (MRI) images and ¹â¸F-fluorodeoxyglucose (FDG) positron-emission tomography (PET) images were acquired using 7.0-T MRI and high-resolution research tomograph FDG-PET, respectively, in 9 early-stage AD patients and 10 healthy subjects. The hippocampal body was divided into three equal parts (anterior, middle, and posterior), and in each part a region of interest (ROI) was drawn over the cornus ammonis (CA)1, CA2/3, CA4/dentate gyrus (DG), and subiculum. The standardized uptake values of the hippocampal subdivisions were calculated for each ROI as ratios relative to the pons standardized uptake value. Statistical analysis was conducted using the Mann-Whitney U test.
RESULTS
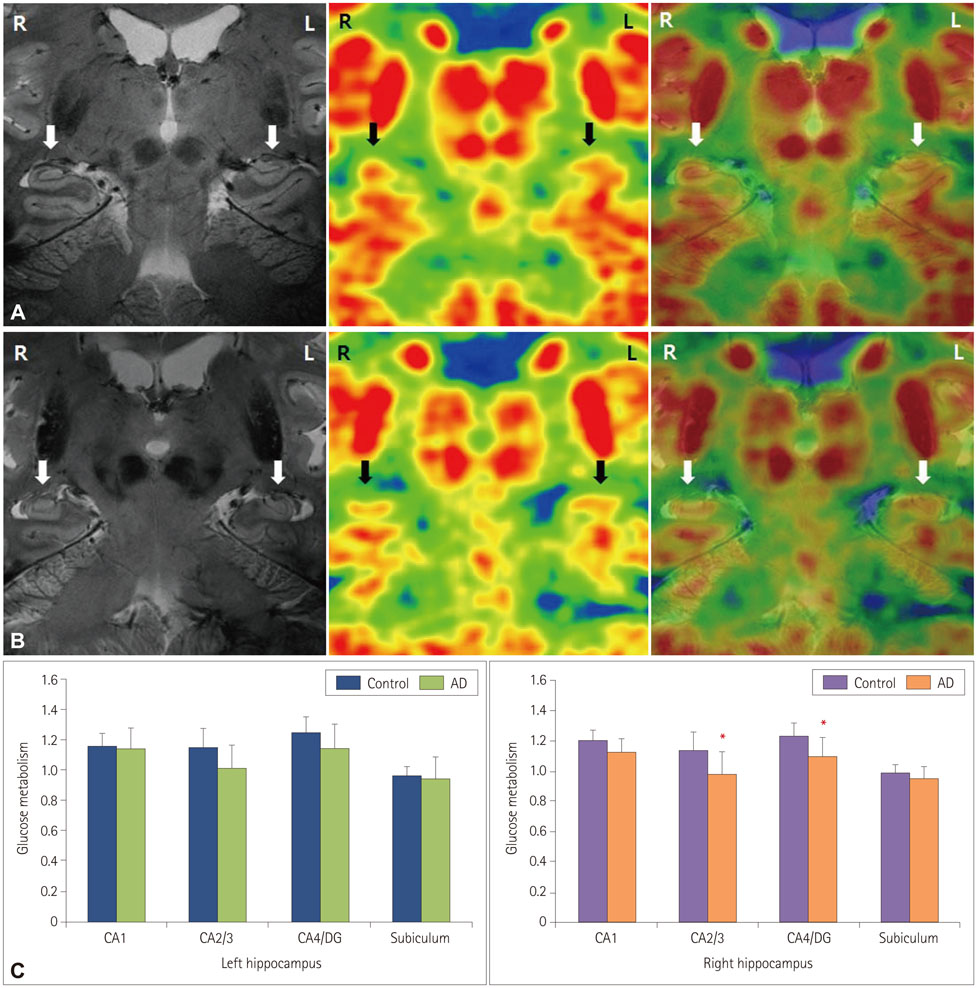
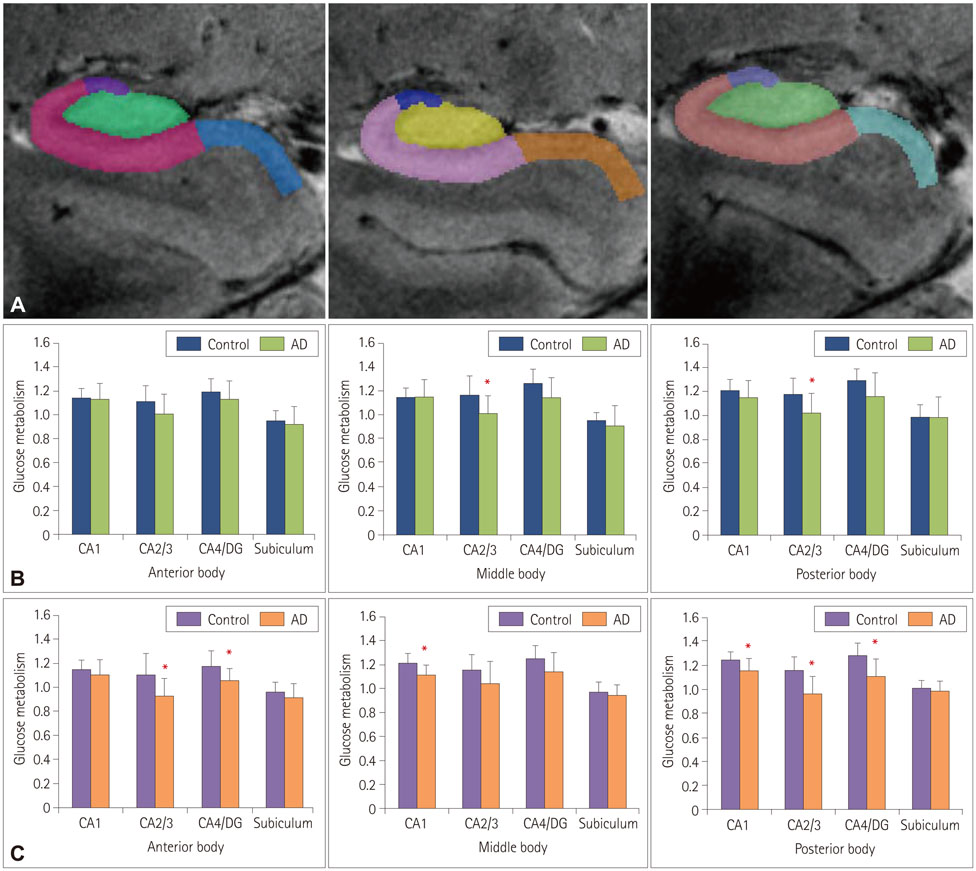
Patients with early-stage AD patients showed significantly less metabolic activity than healthy controls focally in the middle (p=0.050) and posterior (p=0.034) CA2/3 regions of the right hippocampus, and significantly less activity throughout the left hippocampal body in the anterior CA2/3 (p=0.027) and CA4/DG (p=0.027) regions, the middle CA1 region (p=0.011), and the posterior CA1 (p=0.034), CA2/3 (p=0.007), and CA4/DG (p=0.014) regions.
CONCLUSIONS
It was possible to use high-resolution PET-MRI fusion images to identify hippocampus subdivisions and assess glucose metabolism in the subfields. Reductions in metabolic activity were found to vary along the hippocampal axis in early-stage AD patients.
MeSH Terms
Figure
Reference
-
1. Duvernoy HM. The Human Hippocampus: Functional Anatomy, Vascularization and Serial Sections with MRI. 3rd ed. Berlin: Springer-Verlag;2005.2. Kerchner GA, Hess CP, Hammond-Rosenbluth KE, Xu D, Rabinovici GD, Kelley DA, et al. Hippocampal CA1 apical neuropil atrophy in mild Alzheimer disease visualized with 7-T MRI. Neurology. 2010; 75:1381–1387.
Article3. Shastri L. A computational model of episodic memory formation in the hippocampal system. Neurocomputing. 2001; 38:889–897.
Article4. Langston RF, Stevenson CH, Wilson CL, Saunders I, Wood ER. The role of hippocampal subregions in memory for stimulus associations. Behav Brain Res. 2010; 215:275–291.
Article5. Weiner MW, Veitch DP, Aisen PS, Beckett LA, Cairns NJ, Green RC, et al. The Alzheimer's Disease Neuroimaging Initiative: a review of papers published since its inception. Alzheimers Dement. 2012; 8:1 Suppl. S1–S68.
Article6. Theysohn JM, Kraff O, Maderwald S, Schlamann MU, de Greiff A, Forsting M, et al. The human hippocampus at 7 T--in vivo MRI. Hippocampus. 2009; 19:1–7.
Article7. Cho ZH, Han JY, Hwang SI, Kim DS, Kim KN, Kim NB, et al. Quantitative analysis of the hippocampus using images obtained from 7.0 T MRI. Neuroimage. 2010; 49:2134–2140.
Article8. Bobinski M, Wegiel J, Wisniewski HM, Tarnawski M, Reisberg B, Mlodzik B, et al. Atrophy of hippocampal formation subdivisions correlates with stage and duration of Alzheimer disease. Dementia. 1995; 6:205–210.
Article9. Kerchner GA, Deutsch GK, Zeineh M, Dougherty RF, Saranathan M, Rutt BK. Hippocampal CA1 apical neuropil atrophy and memory performance in Alzheimer's disease. Neuroimage. 2012; 63:194–202.
Article10. Herholz K, Herscovitch P, Heiss WD. NeuroPET: Positron Emission Tomography in Neuroscience and Clinical Neurology; with 66 Figures and 11 Tables. Berlin: Springer-Verlag;2004.11. Lewczuk P, Mroczko B, Fagan A, Kornhuber J. Biomarkers of Alzheimer's disease and mild cognitive impairment: a current perspective. Adv Med Sci. 2015; 60:76–82.
Article12. Wienhard K, Schmand M, Casey ME, Baker K, Bao J, Eriksson L, et al. The ECAT HRRT: performance and first clinical application of the new high resolution research tomograph. IEEE Trans Nucl Sci. 2002; 49:104–110.
Article13. Cho ZH, Son YD, Kim HK, Kim ST, Lee SY, Chi JG, et al. Substructural hippocampal glucose metabolism observed on PET/MRI. J Nucl Med. 2010; 51:1545–1548.
Article14. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984; 34:939–944.
Article15. Ahn HJ, Chin J, Park A, Lee BH, Suh MK, Seo SW, et al. Seoul Neuropsychological Screening Battery-dementia version (SNSB-D): a useful tool for assessing and monitoring cognitive impairments in dementia patients. J Korean Med Sci. 2010; 25:1071–1076.
Article16. Cho ZH, Son YD, Kim HK, Kim KN, Oh SH, Han JY, et al. A fusion PET-MRI system with a high-resolution research tomograph-PET and ultra-high field 7.0 T-MRI for the molecular-genetic imaging of the brain. Proteomics. 2008; 8:1302–1323.
Article17. Mueller SG, Stables L, Du AT, Schuff N, Truran D, Cashdollar N, et al. Measurement of hippocampal subfields and age-related changes with high resolution MRI at 4T. Neurobiol Aging. 2007; 28:719–726.
Article18. Schönheit B, Zarski R, Ohm TG. Spatial and temporal relationships between plaques and tangles in Alzheimer-pathology. Neurobiol Aging. 2004; 25:697–711.
Article19. Apostolova LG, Dinov ID, Dutton RA, Hayashi KM, Toga AW, Cummings JL, et al. 3D comparison of hippocampal atrophy in amnestic mild cognitive impairment and Alzheimer's disease. Brain. 2006; 129:2867–2873.
Article20. de Flores R, La Joie R, Chételat G. Structural imaging of hippocampal subfields in healthy aging and Alzheimer's disease. Neuroscience. 2015; 309:29–50.
Article21. Hanseeuw BJ, Van Leemput K, Kavec M, Grandin C, Seron X, Ivanoiu A. Mild cognitive impairment: differential atrophy in the hippocampal subfields. AJNR Am J Neuroradiol. 2011; 32:1658–1661.
Article22. Lim HK, Hong SC, Jung WS, Ahn KJ, Won WY, Hahn C, et al. Automated hippocampal subfield segmentation in amnestic mild cognitive impairments. Dement Geriatr Cogn Disord. 2012; 33:327–333.
Article23. Lim HK, Hong SC, Jung WS, Ahn KJ, Won WY, Hahn C, et al. Automated segmentation of hippocampal subfields in drug-naïve patients with Alzheimer disease. AJNR Am J Neuroradiol. 2013; 34:747–751.
Article24. Yushkevich PA, Pluta JB, Wang H, Xie L, Ding SL, Gertje EC, et al. Automated volumetry and regional thickness analysis of hippocampal subfields and medial temporal cortical structures in mild cognitive impairment. Hum Brain Mapp. 2015; 36:258–287.
Article25. Derflinger S, Sorg C, Gaser C, Myers N, Arsic M, Kurz A, et al. Grey-matter atrophy in Alzheimer's disease is asymmetric but not lateralized. J Alzheimers Dis. 2011; 25:347–357.
Article26. Murray ME, Graff-Radford NR, Ross OA, Petersen RC, Duara R, Dickson DW. Neuropathologically defined subtypes of Alzheimer's disease with distinct clinical characteristics: a retrospective study. Lancet Neurol. 2011; 10:785–796.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neuroimaging Markers for Alzheimer's Disease and Mild Cognitive Impairment in Alzheimer's Disease Neuroimaging Initiative (ADNI)
- ¹â¸F-FDG PET/MR Refines Evaluation in Newly Diagnosed Metastatic Urethral Adenocarcinoma
- Effect of Amyloid Deposition in PET on Hippocampal Metabolism in Amnestic-Mild Cognitive Impairment : Pilot Study
- Neuropsychological and Neuroimaging Findings of Frontal Variant of Alzheimer's Disease
- Role of ¹â¸F-FDG PET-CT in Monitoring the Cyclophosphamide Induced Pulmonary Toxicity in Patients with Breast Cancer: 2 Case Reports