Yonsei Med J.
2018 May;59(3):406-415. 10.3349/ymj.2018.59.3.406.
Effect of Placenta-Derived Mesenchymal Stem Cells in a Dementia Rat Model via Microglial Mediation: a Comparison between Stem Cell Transplant Methods
- Affiliations
-
- 1Department of Neurosurgery, Yonsei University College of Medicine, Seoul, Korea. jchang@yuhs.ac
- 2Brain Korea 21 PLUS Project for Medical Science and Brain Research Institute, Yonsei University College of Medicine, Seoul, Korea.
- 3General Research Institute, Gangnam CHA General Hospital, Seoul, Korea.
- 4Department of Bioengineering, College of Life Science, CHA University, Seoul, Korea.
- KMID: 2407864
- DOI: http://doi.org/10.3349/ymj.2018.59.3.406
Abstract
- PURPOSE
Loss of cholinergic neurons in the hippocampus is a hallmark of many dementias. Administration of stem cells as a therapeutic intervention for patients is under active investigation, but the optimal stem cell type and transplantation modality has not yet been established. In this study, we studied the therapeutic effects of human placenta-derived mesenchymal stem cells (pMSCs) in dementia rat model using either intracerebroventricular (ICV) or intravenous (IV) injections and analyzed their mechanisms of therapeutic action.
MATERIALS AND METHODS
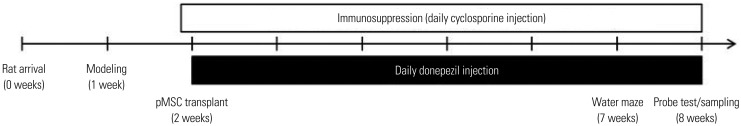
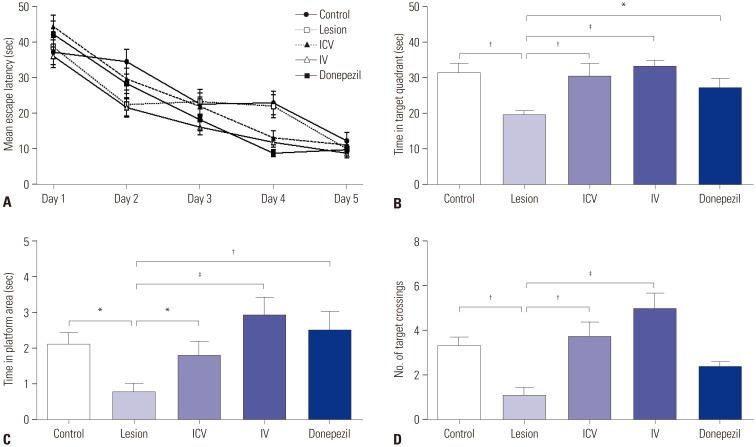
Dementia modeling was established by intraventricular injection of 192 IgG-saporin, which causes lesion of cholinergic neurons. Sixty-five male Sprague-Dawley rats were divided into five groups: control, lesion, lesion+ICV injection of pMSCs, lesion+IV injection of pMSCs, and lesion+donepezil. Rats were subjected to the Morris water maze and subsequent immunostaining analyses.
RESULTS
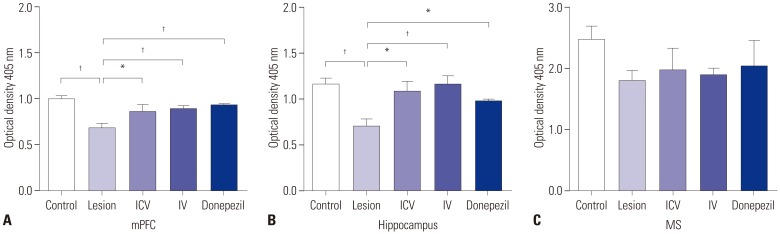
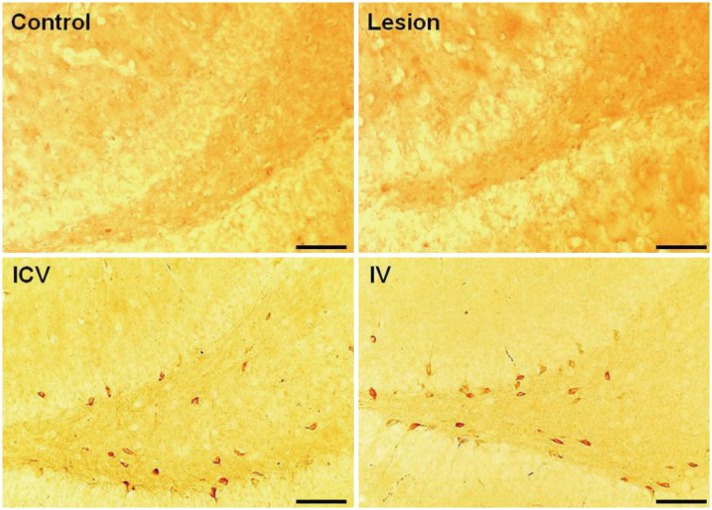
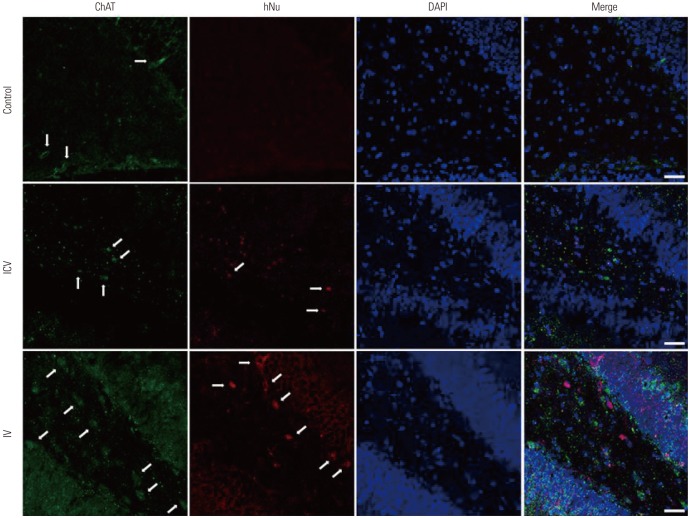
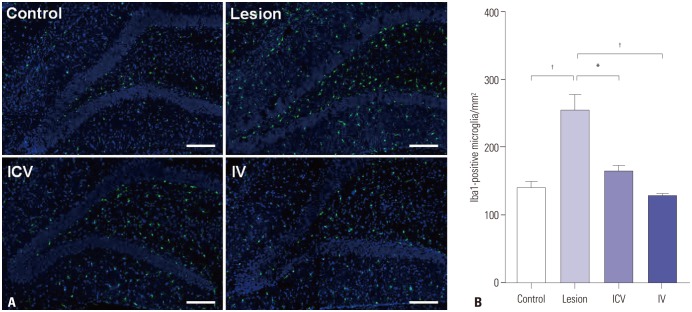
Both ICV and IV pMSC administrations allowed significant cognitive recovery compared to the lesioned rats. Acetylcholinesterase activity was significantly rescued in the hippocampus of rats injected with pMSCs post-lesion. Choline acetyltransferase did not co-localize with pMSCs, showing that pMSCs did not directly differentiate into cholinergic cells. Number of microglial cells increased in lesioned rats and significantly decreased back to normal levels with pMSC injection.
CONCLUSION
Our results suggest that ICV and IV injections of pMSCs facilitate the recovery of cholinergic neuronal populations and cognitive behavior. This recovery likely occurs through paracrine effects that resemble microglia function rather than direct differentiation of injected pMSCs into cholinergic neurons.
MeSH Terms
-
Acetylcholinesterase
Animals
Choline O-Acetyltransferase
Cholinergic Neurons
Dementia*
Hippocampus
Humans
Injections, Intraventricular
Male
Mesenchymal Stromal Cells*
Methods*
Microglia
Models, Animal*
Negotiating*
Placenta
Rats*
Rats, Sprague-Dawley
Stem Cells*
Therapeutic Uses
Water
Acetylcholinesterase
Choline O-Acetyltransferase
Therapeutic Uses
Water
Figure
Reference
-
1. Pappas BA, Bayley PJ, Bui BK, Hansen LA, Thal LJ. Choline acetyltransferase activity and cognitive domain scores of Alzheimer's patients. Neurobiol Aging. 2000; 21:11–17. PMID: 10794843.
Article2. Hall H, Reyes S, Landeck N, Bye C, Leanza G, Double K, et al. Hippocampal Lewy pathology and cholinergic dysfunction are associated with dementia in Parkinson's disease. Brain. 2014; 137:2493–2508. PMID: 25062696.
Article3. Ricceri L, Minghetti L, Moles A, Popoli P, Confaloni A, De Simone R, et al. Cognitive and neurological deficits induced by early and prolonged basal forebrain cholinergic hypofunction in rats. Exp Neurol. 2004; 189:162–172. PMID: 15296846.
Article4. Baxter MG, Bucci DJ, Gorman LK, Wiley RG, Gallagher M. Selective immunotoxic lesions of basal forebrain cholinergic cells: effects on learning and memory in rats. Behav Neurosci. 1995; 109:714–722. PMID: 7576215.
Article5. Gelfo F, Petrosini L, Graziano A, De Bartolo P, Burello L, Vitale E, et al. Cortical metabolic deficits in a rat model of cholinergic basal forebrain degeneration. Neurochem Res. 2013; 38:2114–2123. PMID: 23925861.
Article6. Lehmann O, Grottick AJ, Cassel JC, Higgins GA. A double dissociation between serial reaction time and radial maze performance in rats subjected to 192 IgG-saporin lesions of the nucleus basalis and/or the septal region. Eur J Neurosci. 2003; 18:651–666. PMID: 12911761.
Article7. Tanna T, Sachan V. Mesenchymal stem cells: potential in treatment of neurodegenerative diseases. Curr Stem Cell Res Ther. 2014; 9:513–521. PMID: 25248677.
Article8. Teixeira FG, Carvalho MM, Sousa N, Salgado AJ. Mesenchymal stem cells secretome: a new paradigm for central nervous system regeneration. Cell Mol Life Sci. 2013; 70:3871–3882. PMID: 23456256.
Article9. Suzuki S, Kawamata J, Iwahara N, Matsumura A, Hisahara S, Matsushita T, et al. Intravenous mesenchymal stem cell administration exhibits therapeutic effects against 6-hydroxydopamine-induced dopaminergic neurodegeneration and glial activation in rats. Neurosci Lett. 2015; 584:276–281. PMID: 25449872.
Article10. Katsuda T, Tsuchiya R, Kosaka N, Yoshioka Y, Takagaki K, Oki K, et al. Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Sci Rep. 2013; 3:1197. PMID: 23378928.
Article11. Zhu SF, Zhong ZN, Fu XF, Peng DX, Lu GH, Li WH, et al. Comparison of cell proliferation, apoptosis, cellular morphology and ultrastructure between human umbilical cord and placenta-derived mesenchymal stem cells. Neurosci Lett. 2013; 541:77–82. PMID: 23523648.
Article12. Parolini O, Soncini M. Placenta as a source of stem cells and as a key organ for fetomaternal tolerance. In : Bhattacharya N, Stubblefield P, editors. Regenerative medicine using pregnancy-specific biological substances. London: Springer London;2011. p. 11–23.13. Parolini O, Alviano F, Bagnara GP, Bilic G, Buhring HJ, Evangelista M, et al. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international workshop on placenta derived stem cells. Stem Cells. 2008; 26:300–311. PMID: 17975221.
Article14. Kadam S, Muthyala S, Nair P, Bhonde R. Human placenta-derived mesenchymal stem cells and islet-like cell clusters generated from these cells as a novel source for stem cell therapy in diabetes. Rev Diabet Stud. 2010; 7:168–182. PMID: 21060975.
Article15. Lee JK, Jin HK, Bae JS. Bone marrow-derived mesenchymal stem cells reduce brain amyloid-beta deposition and accelerate the activation of microglia in an acutely induced Alzheimer's disease mouse model. Neurosci Lett. 2009; 450:136–141. PMID: 19084047.16. Greter M, Merad M. Regulation of microglia development and homeostasis. Glia. 2013; 61:121–127. PMID: 22927325.
Article17. Boscia F, Esposito CL, Di Crisci A, de Franciscis V, Annunziato L, Cerchia L. GDNF selectively induces microglial activation and neuronal survival in CA1/CA3 hippocampal regions exposed to NMDA insult through Ret/ERK signalling. PLoS One. 2009; 4:e6486. PMID: 19649251.
Article18. Lambertsen KL, Clausen BH, Babcock AA, Gregersen R, Fenger C, Nielsen HH, et al. Microglia protect neurons against ischemia by synthesis of tumor necrosis factor. J Neurosci. 2009; 29:1319–1330. PMID: 19193879.
Article19. Kim KS, Kim HS, Park JM, Kim HW, Park Mk, Lee HS, et al. Long-term immunomodulatory effect of amniotic stem cells in an Alzheimer’s disease model. Neurobiol Aging. 2013; 34:2408–2420. PMID: 23623603.
Article20. Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961; 7:88–95. PMID: 13726518.
Article21. Nilsson OG, Strecker RE, Daszuta A, Björklund A. Combined cholinergic and serotonergic denervation of the forebrain produces severe deficits in a spatial learning task in the rat. Brain Res. 1988; 453:235–246. PMID: 3401761.
Article22. Chen TJ, Chen SS, Wang DC, Hung HS. The cholinergic signaling responsible for the expression of a memory-related protein in primary rat cortical neurons. J Cell Physiol. 2016; 231:2428–2438. PMID: 26895748.
Article23. Tatebayashi Y, Lee MH, Li L, Iqbal K, Grundke-Iqbal I. The dentate gyrus neurogenesis: a therapeutic target for Alzheimer’s disease. Acta Neuropathol. 2003; 105:225–232. PMID: 12557008.
Article24. Seaberg RM, van der Kooy D. Adult rodent neurogenic regions: the ventricular subependyma contains neural stem cells, but the dentate gyrus contains restricted progenitors. J Neurosci. 2002; 22:1784–1793. PMID: 11880507.
Article25. Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci U S A. 1999; 96:10711–10716. PMID: 10485891.
Article26. Lee SH, Jin KS, Bang OY, Kim BJ, Park SJ, Lee NH, et al. Differential migration of mesenchymal stem cells to ischemic regions after middle cerebral artery occlusion in rats. PLoS One. 2015; 10:e0134920. PMID: 26241653.
Article27. Lu D, Mahmood A, Wang L, Li Y, Lu M, Chopp M. Adult bone marrow stromal cells administered intravenously to rats after traumatic brain injury migrate into brain and improve neurological outcome. Neuroreport. 2001; 12:559–563. PMID: 11234763.
Article28. Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009; 18:683–692. PMID: 19099374.
Article29. Hamisha KN, Tfilin M, Yanai J, Turgeman G. Mesenchymal stem cells can prevent alterations in behavior and neurogenesis induced by Aß25-35 administration. J Mol Neurosci. 2014; 55:1006–1013. PMID: 25384918.
Article30. Zilka N, Zilkova M, Kazmerova Z, Sarissky M, Cigankova V, Novak M. Mesenchymal stem cells rescue the Alzheimer’s disease cell model from cell death induced by misfolded truncated tau. Neuroscience. 2011; 193:330–337. PMID: 21763758.
Article31. Yoo SW, Chang DY, Lee HS, Kim GH, Park JS, Ryu BY, et al. Immune following suppression mesenchymal stem cell transplantation in the ischemic brain is mediated by TGF-β. Neurobiol Dis. 2013; 58:249–257. PMID: 23759293.
Article32. Kettenmann H, Kirchhoff F, Verkhratsky A. Microglia: new roles for the synaptic stripper. Neuron. 2013; 77:10–18. PMID: 23312512.
Article33. Neher JJ, Neniskyte U, Zhao JW, Bal-Price A, Tolkovsky AM, Brown GC. Inhibition of microglial phagocytosis is sufficient to prevent inflammatory neuronal death. J Immunol. 2011; 186:4973–4983. PMID: 21402900.
Article34. Neumann H, Kotter MR, Franklin RJ. Debris clearance by microglia: an essential link between degeneration and regeneration. Brain. 2009; 132(Pt 2):288–295. PMID: 18567623.
Article35. Sierra A, Abiega O, Shahraz A, Neumann H. Janus-faced microglia: beneficial and detrimental consequences of microglial phagocytosis. Front Cell Neurosci. 2013; 7:6. PMID: 23386811.
Article36. Cunningham CL, Martínez-Cerdeño V, Noctor SC. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J Neurosci. 2013; 33:4216–4233. PMID: 23467340.
Article37. Batchelor PE, Liberatore GT, Wong JY, Porritt MJ, Frerichs F, Donnan GA, et al. Activated macrophages and microglia induce dopaminergic sprouting in the injured striatum and express brainderived neurotrophic factor and glial cell line-derived neurotrophic factor. J Neurosci. 1999; 19:1708–1716. PMID: 10024357.
Article38. Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005; 438:1017–1021. PMID: 16355225.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro
- Mesenchymal Stem Cell Therapy in Pulmonary Disease
- Immunomodulatory Effects of Placenta-derived Mesenchymal Stem Cells on T Cells by Regulation of FoxP3 Expression
- Advanced Research on Stem Cell Therapy for Hepatic Diseases: Potential Implications of a Placenta-derived Mesenchymal Stem Cell-based Strategy
- Upregulation of C-Reactive Protein by Placenta-Derived Mesenchymal Stem Cells Promotes Angiogenesis in A Rat Model with Cirrhotic Liver