Korean J Gastroenterol.
2018 Mar;71(3):132-142. 10.4166/kjg.2018.71.3.132.
Combined Extracts of Artemisia and Green Tea, Mitigated Alcoholic Gastritis Via Enhanced Heat-shock Protein 27
- Affiliations
-
- 1Department of Biochemistry and Molecular Biology, Hanyang University College of Medicine, Seoul, Korea.
- 2CHA Cancer Preventive Research Center, CHA Bio Complex, College of Medicine, CHA University, Pangyo, Korea. hahmkb@cha.ac.kr
- 3S&D Research Center, S&D Foods, Cheongwon, Korea.
- 4Digestive Disease Center, CHA University Bundang Medical Center, College of Medicine, CHA University, Seongnam, Korea.
- KMID: 2407670
- DOI: http://doi.org/10.4166/kjg.2018.71.3.132
Abstract
- BACKGROUND/AIMS
Several lines of evidence from epidemiologic and laboratory studies have shown that the consumption of Artemisia or green tea extracts (MPGT) is inversely associated with the risk of alcohol-induced damage and other chronic diseases. Supported by previous studies showing that the combined extract of Artemisia and green tea, MPGT, exerted significantly either antioxidative or anti-inflammatory actions against Helicobacter pylori-associated gastric diseases, it was hypothesized that MPGT can offer protection against alcoholic gastritis.
METHODS
Ethanol was administered to induce gastric damage in Wistar rats, which had been pretreated with various doses of MPGT, to measure the rescuing action of a MPGT pretreatment against ethanol-induced gastric damage. In addition, the molecular mechanisms for the preventive effects were examined.
RESULTS
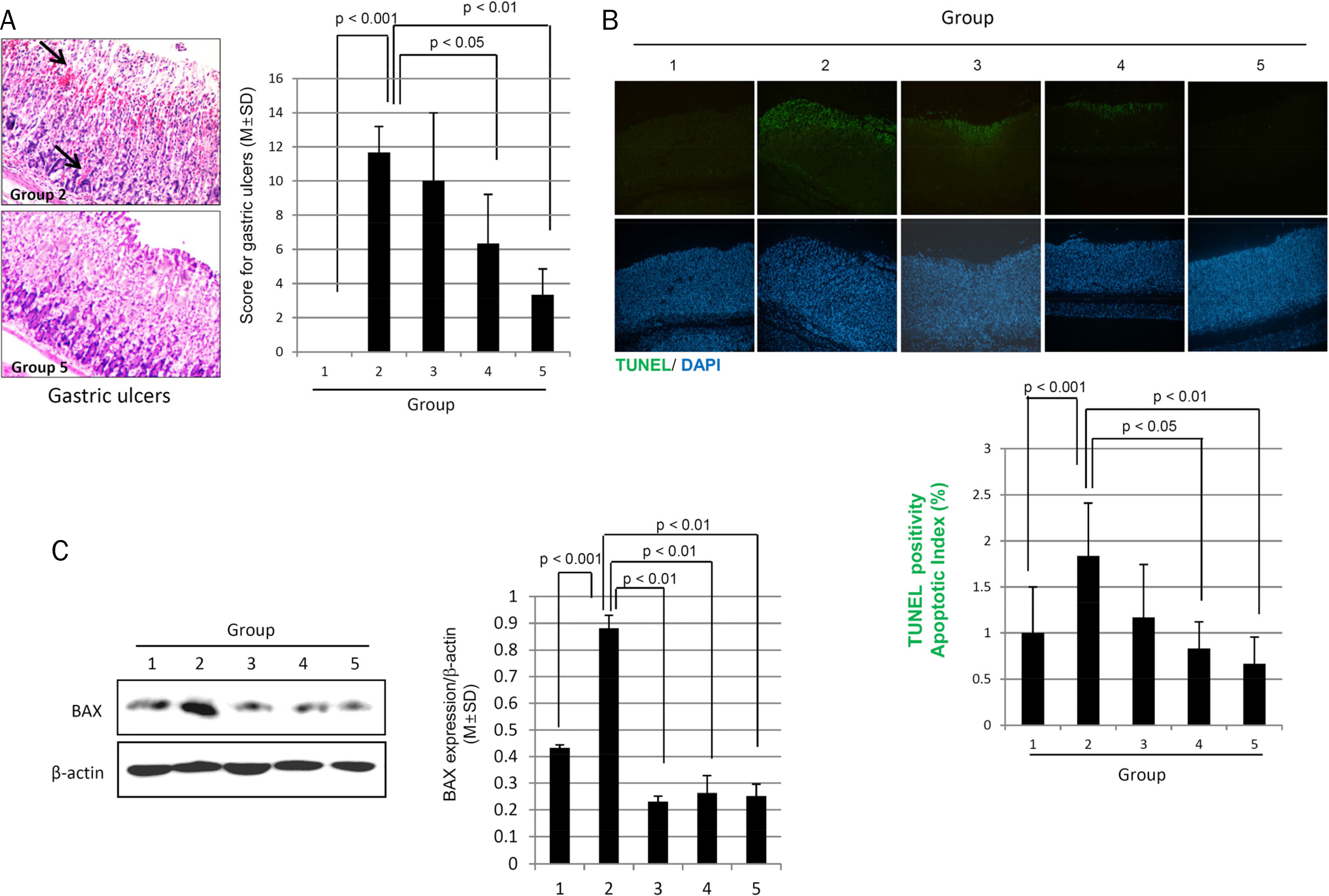
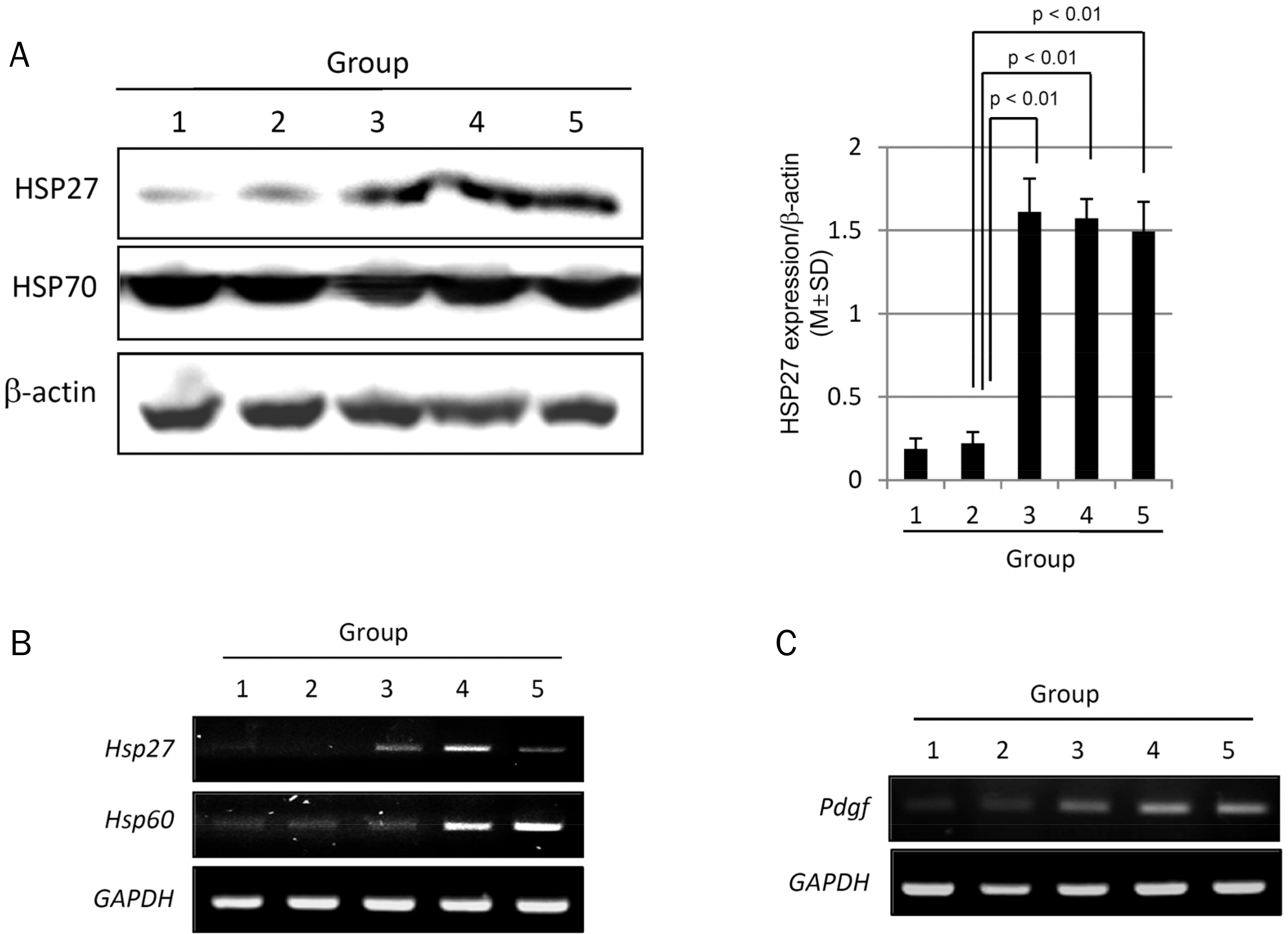
The MPGT pretreatment (100, 300, and 500 mg/kg) alleviated the ethanol-induced gastric damage, which was evidenced by the significant decrease in calcium-dependent phospholipase A2, MAPKs, and NF-κB levels compared to ethanol alone. Furthermore, the MPGT pretreatment preserved 15-prostaglandin dehydrogenase, whereas cyclooxygenase-2 was decreased significantly. All of these biochemical changes led to the significant alleviation of alcohol-associated gastric mucosal damage. Ethanol significantly increased the TUNEL positivity in the stomach, but MPGT decreased the apoptotic index significantly, which was associated with significantly lower pathological scores of ethanol-induced mucosal ulcerations. The significant protective changes observed alcoholic gastritis with MPGT were related to the increased expression of cytoprotective genes, such as heat-shock protein (HSP)27, HSP60, and PDGF.
CONCLUSIONS
The efficient anti-inflammatory, anti-apoptotic, and regenerative actions of MPGT make it a potential nutrient phytoceutical to rescue the stomach from alcoholic gastritis.
Keyword
MeSH Terms
-
Alcoholics*
Artemisia*
Chronic Disease
Cyclooxygenase 2
Ethanol
Gastritis*
Heat-Shock Proteins*
Helicobacter
HSP27 Heat-Shock Proteins*
Humans
In Situ Nick-End Labeling
Oxidoreductases
Phospholipases A2
Rats, Wistar
Stomach
Stomach Diseases
Tea*
Ulcer
Cyclooxygenase 2
Ethanol
HSP27 Heat-Shock Proteins
Heat-Shock Proteins
Oxidoreductases
Phospholipases A2
Tea
Figure
Reference
-
References
1. Hernández-Muñoz R, Montiel-Ruíz C, Vázquez-Martínez O. Gastric mucosal cell proliferation in ethanol-induced chronic abdominal injury is related to oxidative stress and lipid peroxidation in rats. Lab Invest. 2000; 80:1161–1169.2. Kwiecień S, Brzozowski T, Konturek SJ. Effects of reactive oxygen species action on gastric mucosa in various models of mucosal injury. J Physiol Pharmacol. 2002; 53:39–50.3. Park SW, Oh TY, Kim YS, et al. Artemisia asiatica extracts protect against ethanol-induced injury in gastric mucosa of rats. J Gastroenterol Hepatol. 2008; 23:976–984.
Article4. Park JM, Hahm KB, Kwon SO, Kim EH. The anti-inflammatory abdominals of acidic polysaccharide from Artemisia capillaris on Helicobacter pylori infection. J Cancer Prev. 2013; 18:161–168.5. Park JM, Han YM, Lee JS, et al. Nrf2-mediated mucoprotective and anti-inflammatory actions of Artemisia extracts led to at-tenuate stress related mucosal damages. J Clin Biochem Nutr. 2015; 56:132–142.6. Kim YS, Lee HJ, Park JM, et al. Targeted molecular ablation of abdominal stem cells for curing gastrointestinal cancers. Expert Rev Gastroenterol Hepatol. 2017; 11:1059–1070.7. Kuzuhara T, Suganuma M, Fujiki H. Green tea catechin as a chemical chaperone in cancer prevention. Cancer Lett. 2008; 261:12–20.
Article8. Jeong M, Park JM, Han YM, et al. Dietary intervention of artemisia and green tea extracts to rejuvenate Helicobacter pyloriassociated chronic atrophic gastritis and to prevent tumorigenesis. Helicobacter. 2016; 21:40–59.9. Nam SY, Kim N, Lee CS, et al. Gastric mucosal protection via abdominal of MUC5AC and MUC6 by geranylgeranylacetone. Dig Dis Sci. 2005; 50:2110–2120.10. Goo MJ, Ki MR, Lee HR, et al. Helicobacter pylori promotes abdominal fibrosis in the animal model. Lab Invest. 2009; 89:1291–1303.11. Linkous A, Yazlovitskaya E. Cytosolic phospholipase A2 as a abdominal of disease pathogenesis. Cell Microbiol. 2010; 12:1369–1377.12. Pandey R, Ghorpade A. Cytosolic phospholipase A2 regulates al-cohol-mediated astrocyte inflammatory responses in HIV-abdominal neurocognitive disorders. Cell Death Discov. 2015; 1:15045.
Article13. Kang GD, Lee SY, Jang SE, Han MJ, Kim DH. Irisolidone abdominal ethanol-induced gastric injury in mice by inhibiting the abdominal of neutrophils. Mol Nutr Food Res. 2017; 61.14. Song HJ, Myung SJ, Kim IW, et al. 15-hydroxyprostaglandin abdominal is downregulated and exhibits tumor suppressor activity in gastric cancer. Cancer Invest. 2011; 29:257–265.15. Jiang M, Gao PF, Li HQ, Tian PY, Fan XM. Ghrelin inhibition of abdominal-induced gastric epithelial cell apoptosis is mediated by miR-21. Int J Clin Exp Pathol. 2015; 8:4662–4672.16. El-Maraghy SA, Rizk SM, Shahin NN. Gastroprotective effect of crocin in ethanol-induced gastric injury in rats. Chem Biol Interact. 2015; 229:26–35.
Article17. Antonisamy P, Subash-Babu P, Alshatwi AA, et al. Gastroprotective effect of nymphayol isolated from Nymphaea stellata (Willd.) flowers: contribution of antioxidant, anti-inflammatory and anti-apoptotic activities. Chem Biol Interact. 2014; 224:157–163.
Article18. Rozza AL, Meira de Faria F, Souza Brito AR, Pellizzon CH. The abdominal effect of menthol: involvement of anti-apoptotic, antioxidant and anti-inflammatory activities. PLoS One. 2014; 9:e86686.19. Luiz-Ferreira A, Cola M, Barbastefano V, et al. Healing, abdominal and cytoprotective properties of indigofera truxillensis in different models of gastric ulcer in rats. Int J Mol Sci. 2012; 13:14973–14991.20. Saika M, Ueyama T, Senba E. Expression of immediate early genes, HSP70, and COX-2 mRNAs in rat stomach following abdominal ingestion. Dig Dis Sci. 2000; 45:2455–2462.21. Lee JS, Oh TY, Kim YK, et al. Protective effects of green tea poly-phenol extracts against ethanol-induced gastric mucosal damages in rats: stress-responsive transcription factors and MAP abdominal as potential targets. Mutat Res. 2005; 579:214–224.22. Ozbayer C, Kurt H, Ozdemir Z, et al. Gastroprotective, abdominal and antioxidant effects of oleum cinnamomi on ethanol induced damage. Cytotechnology. 2014; 66:431–441.23. Al Batran R, Al-Bayaty F, Jamil Al-Obaidi MM, et al. In vivo abdominal and antiulcer activity of Parkia speciosa ethanolic leaf extract against ethanol-induced gastric ulcer in rats. PLoS One. 2013; 8:e64751.24. Ibrahim IA, Qader SW, Abdulla MA, Nimir AR, Abdelwahab SI, Al-Bayaty FH. Effects of Pithecellobium jiringa ethanol extract against ethanol-induced gastric mucosal injuries in Sprague-Dawley rats. Molecules. 2012; 17:2796–2811.
Article25. Ibrahim IA, Abdulla MA, Hajrezaie M, et al. The gastroprotective effects of hydroalcoholic extract of Monolluma quadrangula against ethanol-induced gastric mucosal injuries in Sprague Dawley rats. Drug Des Devel Ther. 2016; 10:93–105.
Article26. Lee HJ, Ock CY, Kim SJ, Hahm KB. Heat shock protein: hard work-er or bad offender for gastric diseases. Int J Proteomics. 2010; 2010:259163.
Article27. Choi SR, Lee SA, Kim YJ, Ok CY, Lee HJ, Hahm KB. Role of heat shock proteins in gastric inflammation and ulcer healing. J Physiol Pharmacol. 2009; 60(Suppl 7):5–17.28. Yeo M, Park HK, Kim DK, et al. Restoration of heat shock protein70 suppresses gastric mucosal inducible nitric oxide abdominal expression induced by Helicobacter pylori. Proteomics. 2004; 4:3335–3342.29. Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci. 2005; 62:670–684.
Article30. Salaspuro M. Acetaldehyde and gastric cancer. J Dig Dis. 2011; 12:51–59.
Article31. Hellström PM, Hendolin P, Kaihovaara P, et al. Slow-release L-cys-teine capsule prevents gastric mucosa exposure to carcinogenic acetaldehyde: results of a randomised single-blinded, abdominal study of Helicobacterassociated atrophic gastritis. Scand J Gastroenterol. 2017; 52:230–237.32. Lee HJ, Park JM, Han YM, et al. The role of chronic inflammation in the development of gastrointestinal cancers: reviewing cancer prevention with natural anti-inflammatory intervention. Expert Rev Gastroenterol Hepatol. 2016; 10:129–139.
Article33. Tai HH. Prostaglandin catabolic enzymes as tumor suppressors. Cancer Metastasis Rev. 2011; 30:409–417.
Article34. Na HK, Park JM, Lee HG, Lee HN, Myung SJ, Surh YJ. 15-Hydroxyprostaglandin dehydrogenase as a novel molecular target for cancer chemoprevention and therapy. Biochem Pharmacol. 2011; 82:1352–1360.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Transactivation of peroxisome proliferator-activated receptor alpha by green tea extracts
- Expression of Heat Shock Protein 70 m-RNA in Rat Bladder Overdistended by Diuresis
- Environmental factors regulating the expression of Porphyromonas gingivalis heat shock protein
- Randomized, Double-blind, Placebo-controlled Clinical Trial for the Evaluation of the Efficacy and Safety of Artemisia and Green Tea Extract SD1003F in Volunteers with Helicobacter pylori-associated Gastric Discomfort
- The Protective Effect of Induced Heat Shock Protein in Human Corneal Epithelial Cells