J Vet Sci.
2018 Mar;19(2):280-289. 10.4142/jvs.2018.19.2.280.
Haemoproteus in barn and collared scops owls from Thailand
- Affiliations
-
- 1Department of Pathology, Faculty of Veterinary Medicine, Kasetsart University Kamphaeng Saen Campus, Nakorn Pathom 73140, Thailand. fvetcls@ku.ac.th
- 2Department of Pathology, Faculty of Veterinary Medicine, Kasetsart University, Bangkok 10903, Thailand.
- 3Kasetsart University Raptor Rehabilitation Unit, Faculty of Veterinary Medicine, Kasetsart University Kamphaeng Saen Campus, Nakorn Pathom 73140, Thailand.
- 4Department of Large Animal and Wildlife Clinical Sciences, Faculty of Veterinary Medicine, Kasetsart University Kamphaeng Saen Campus, Nakorn Pathom 73140, Thailand.
- KMID: 2407627
- DOI: http://doi.org/10.4142/jvs.2018.19.2.280
Abstract
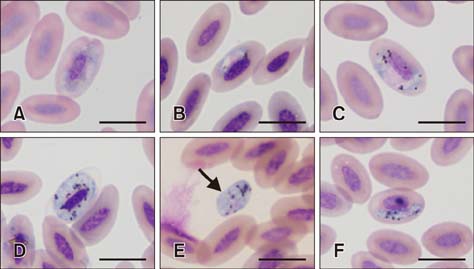
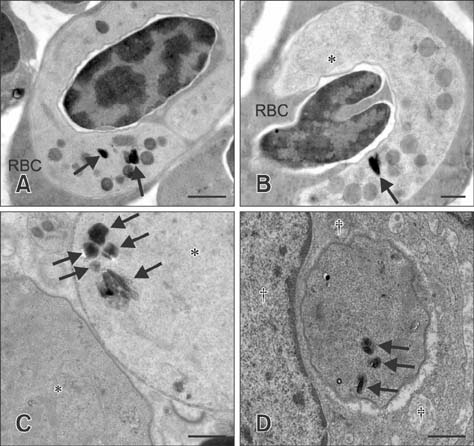
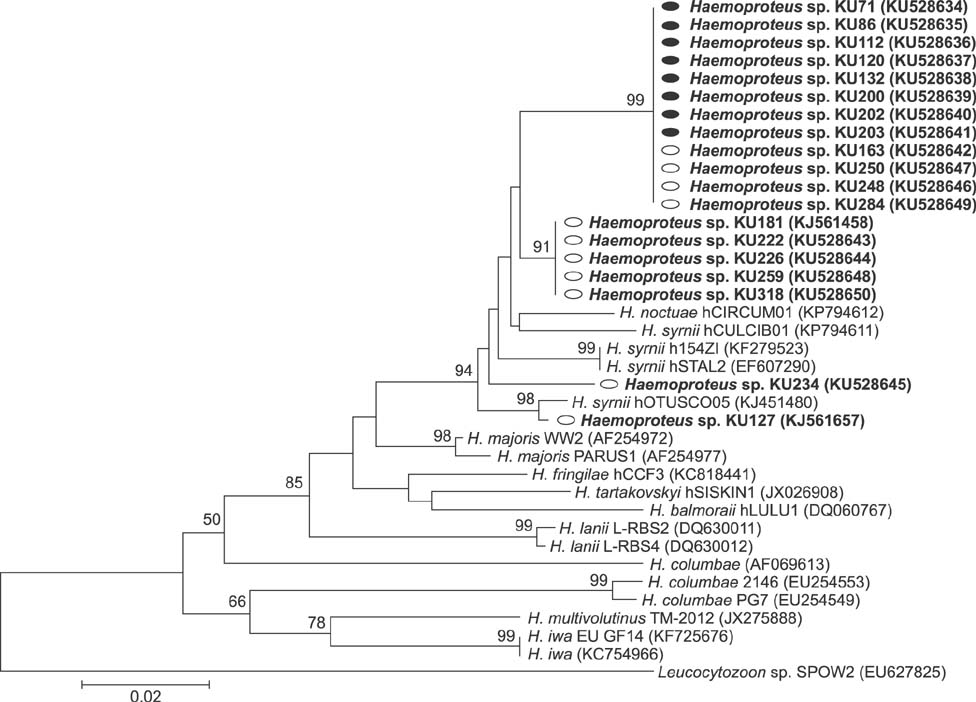
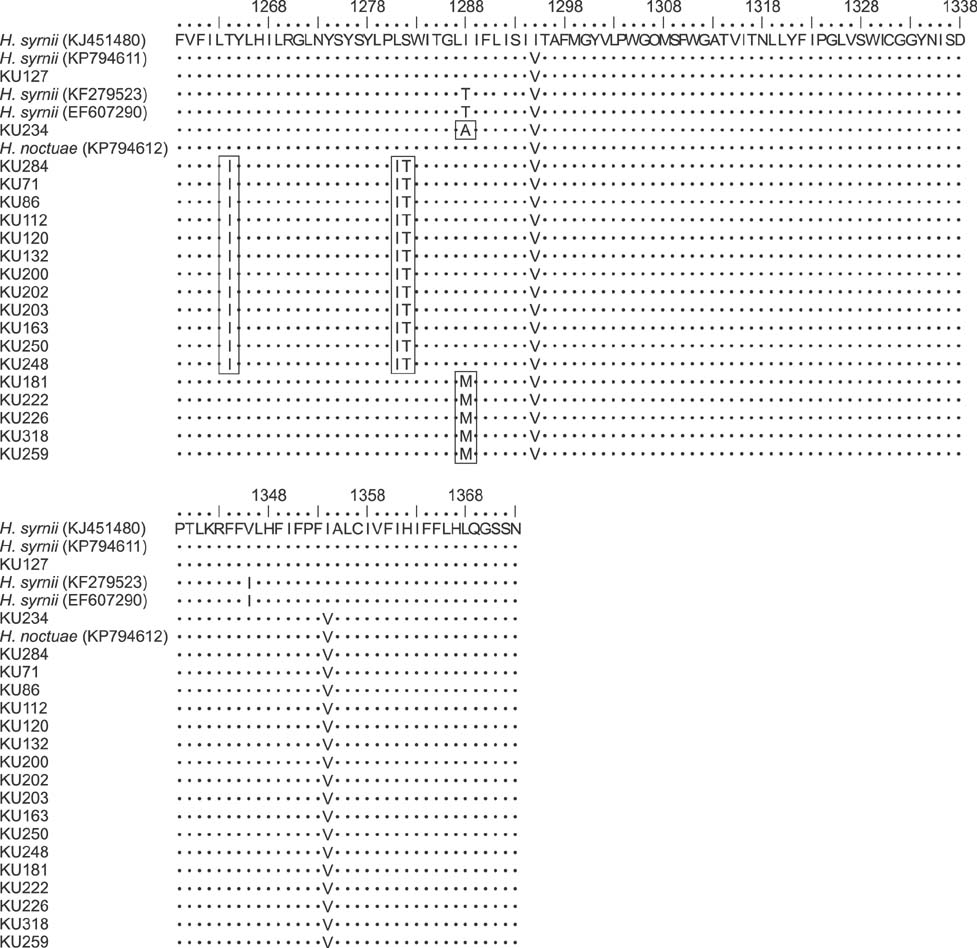
- The barn owl (BO) and the collared scops owl (CSO) are common nocturnal raptors throughout Thailand. Blood samples from 23 adult BOs and 14 CSOs were collected and processed for complete blood cell counts and parasite morphological examinations. Two Haemoproteus-positive samples were processed for ultrastructural observation. Polymerase chain reaction (PCR) analysis for a partial cytochrome b gene (cytb) from Haemoproteus was performed in all samples. Haemoproteus presence detected by light microscopy was lower than that detected by PCR (30.4% and 34.8%, respectively, in BO; and 50.0% and 78.6%, respectively, in CSO). Comparative hematology revealed that Haemoproteus-positive BOs had higher mean cell hemoglobin concentration, total leukocyte, absolute heterophil, basophil, and monocyte counts than Haemoproteus-negative BOs, but no significant differences between Haemoproteus-negative and -positive CSOs. Monocyte ultrastructure analysis revealed a role in the elimination of gametocytes. Morphologically, the Haemoproteus in 3 BOs and 6 CSOs were identified as H. noctuae, while that in 1 CSO was identified as H. syrnii. Phylogenetic analysis indicated the Haemoproteus spp. in 8 BOs and 7 CSOs were not closely related to H. noctuae or H. syrnii, and the cytb of 2 CSOs was that of H. syrnii. These results should be useful for study of Haemoproteus.
Keyword
MeSH Terms
Figure
Reference
-
1. Ammersbach M, Beaufrère H, Gionet Rollick A, Tully T. Laboratory blood analysis in Strigiformes---Part I: hematologic reference intervals and agreement between manual blood cell counting techniques. Vet Clin Pathol. 2015; 44:94–108.
Article2. Bennett GF, Campbell AG. Avian Haemoproteidae. I. Description of Haemoproteus fallisi n. sp. and a review of the haemoproteids of the family Turdidae. Can J Zool. 1972; 50:1269–1275.
Article3. Bennett GF, Peirce MA. Morphological form in the avian Haemoproteidae and an annotated checklist of the genus Haemoproteus Kruse, 1890. J Natural History. 1988; 22:1683–1696.
Article4. Bukauskaitė D, Žiegytė R, Palinauskas V, Iezhova TA, Dimitrov D, Ilgūnas M, Bernotienė R, Markovets MY, Valkiūnas G. Biting midges (Culicoides, Diptera) transmit Haemoproteus parasites of owls: evidence from sporogony and molecular phylogeny. Parasit Vectors. 2015; 8:303.
Article5. Campbell T, Ellis C. Hematology of birds. In : Campbell TW, Ellis C, editors. Avian and Exotic Animal Hematology and Cytology. 3rd ed. Oxford: Wiley-Blackwell;2007. p. 3–50.6. Cosgrove CL, Knowles SC, Day KP, Sheldon BC. No evidence for avian malaria infection during the nestling phase in a passerine bird. J Parasitol. 2006; 92:1302–1304.
Article7. del Hoyo J, Elliott A, Sargatal J. Handbook of the Birds of the World: Barn-Owls to Hummingbirds. 5. Barcelona: Lynx Edicions;1999. p. 34–158.8. Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser. 1999; 41:95–98.9. Hellgren O, Krizanauskiene A, Valkĭunas G, Bensch S. Diversity and phylogeny of mitochondrial cytochrome b lineages from six morphospecies of avian Haemoproteus (Haemosporida: Haemoproteidae). J Parasitol. 2007; 93:889–896.
Article10. Hellgren O, Waldenström J, Bensch S. A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avian blood. J Parasitol. 2004; 90:797–802.
Article11. Ishtiaq F, Gering E, Rappole JH, Rahmani AR, Jhala YV, Dove CJ, Milensky C, Olson SL, Peirce MA, Fleischer RC. Prevalence and diversity of avian hematozoan parasites in Asia: a regional survey. J Wildl Dis. 2007; 43:382–398.
Article12. Karadjian G, Martinsen E, Duval L, Chavatte JM, Landau I. Haemoproteus ilanpapernai n. sp. (Apicomplexa, Haemoproteidae) in Strix seloputo from Singapore: morphological description and reassignment of molecular data. Parasite. 2014; 21:17.
Article13. Karadjian G, Puech MP, Duval L, Chavatte JM, Snounou G, Landau I. Haemoproteus syrnii in Strix aluco from France: morphology, stages of sporogony in a hippoboscid fly, molecular characterization and discussion on the identification of Haemoproteus species. Parasite. 2013; 20:32.
Article14. Kidsin K, Sanyathitiseree P, Pothieng D, Wajjwalku W, Kasorndorkbua C. A retrospective study of morbidity and mortality of raptors in Kasetsart University Raptor Rehabilitation Unit, 2008–2011. Korean J Ornithol. 2012; 19:87–92.15. Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980; 16:111–120.
Article16. Krizanauskiene A, Pérez-Tris J, Palinauskas V, Hellgren O, Bensch S, Valkiūnas G. Molecular phylogenetic and morphological analysis of haemosporidian parasites (Haemosporida) in a naturally infected European songbird, the blackcap Sylvia atricapilla, with description of Haemoproteus pallidulus sp. nov. Parasitology. 2010; 137:217–227.
Article17. Krone O, Priemer J, Streich J, Sömmer P, Langgemach T, Lessow O. Haemosporida of birds of prey and owls from Germany. Acta Protozool. 2001; 40:281–289.18. Krone O, Waldenström J, Valkiūnas G, Lessow O, Müller K, Iezhova TA, Fickel J, Bensch S. Haemosporidian blood parasites in European birds of prey and owls. J Parasitol. 2008; 94:709–715.
Article19. Lacasse C. Falconiformes (falcons, hawks, eagles, kites, harriers, bazzards, ospreys, caracaras, secretary birds, old world and new world vultures). In : Miller RE, Fowler ME, editors. Fowler's Zoo and Wild Animal Medicine. St. Louis: Elsevier-Saunders;2012. p. 127–142.20. Mutlow A, Forbes N. Haemoproteus in raptor: pathogenicity, treatment and control. In : Proceedings of the 21st Annual Conference and Expo of the Association of Avian Veterinarians; 30 August-1 September 2000; Portland, USA. p. 157–163.21. Paperna I, Keong MSC, May CYA. Haemosporozoan parasites found in birds in Peninsular Malaysia, Singapore, Sarawak and Java. Raffles Bull Zool. 2008; 56:211–243.22. Remple JD. Intracellular hematozoa of raptors: a review and update. J Avian Med Surg. 2004; 18:75–88.
Article23. Salakij C, Kasorndorkbua C, Salakij J, Suwannasaeng P, Jakthong P. Quantitative and qualitative morphologic, cytochemical and ultrastructural characteristics of blood cells in the Crested Serpent eagle and Shikra. Jpn J Vet Res. 2015; 63:95–105.24. Salakij J, Lertwatcharasarakul P, Kasorndorkbua C, Salakij C. Plasmodium circumflexum in a Shikra (Accipiter badius): phylogeny and ultra-structure of the haematozoa. Jpn J Vet Res. 2012; 60:105–109.25. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular Evolutionary Genetics Analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011; 28:2731–2739.
Article26. Valkiūnas G. Avian Malaria Parasites and Other Haemosporidia. Boca Raton: CRC Press;2005. p. 1–268.27. Valkiūnas G, Iezhova TA, Krizanauskiene A, Palinauskas V, Sehgal RN, Bensch S. A comparative analysis of microscopy and PCR-based detection methods for blood parasites. J Parasitol. 2008; 94:1395–1401.
Article28. Valkiūnas G, Iezhova TA, Loiseau C, Sehgal RN. Nested cytochrome b polymerase chain reaction diagnostics detect sporozoites of hemosporidian parasites in peripheral blood of naturally infected birds. J Parasitol. 2009; 95:1512–1515.
Article29. Valkiūnas G, Palinauskas V, Ilgūnas M, Bukauskaitė D, Dimitrov D, Bernotienė R, Zehtindjiev P, Ilieva M, Iezhova TA. Molecular characterization of five widespread avian haemosporidian parasites (Haemosporida), with perspectives on the PCR-based detection of haemosporidians in wildlife. Parasitol Res. 2014; 113:2251–2263.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects on hematology and blood biochemistry profile of intramuscular meloxicam injection in Brahminy kite and Barn owl
- The Growing Problem of Radiologist Shortage: Thailand’s Perspective
- Perspectives on veterinary education in Thailand
- SIMULTANEOUS RECONSTRUCTION AFTER RADIAL SINUSECTOMY IN PATIENTS WITH A LARGE ETHMOID AND MAXILLARY MUCOCELE
- Occupational asthma induced by tobacco leaf