Cancer Res Treat.
2018 Jan;50(1):50-59. 10.4143/crt.2017.027.
Risk and Characteristics of Postcolonoscopy Interval Colorectal Cancer after a Positive Fecal Test: A Nationwide Population-Based Study in Korea
- Affiliations
-
- 1Department of Internal Medicine, Kyung Hee University School of Medicine, Seoul, Korea.
- 2Department of Cancer Control and Policy, Graduate School of Cancer Science and Policy, National Cancer Center, Goyang, Korea. kschoi@ncc.re.kr
- 3Department of Internal Medicine, Hanyang University Guri Hospital, Hanyang University College of Medicine, Guri, Korea. hands@hanyang.ac.kr
- 4Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 5National Cancer Control Institute, National Cancer Center, Goyang, Korea.
- KMID: 2403474
- DOI: http://doi.org/10.4143/crt.2017.027
Abstract
- PURPOSE
Fecal tests remain a mainstay of population-based colorectal cancer (CRC) screening programs worldwide. However, data on interval CRC (iCRC) arising after follow-up colonoscopy of a positive fecal test are scarce. We conducted a nationwide population-based study to reveal the risk and characteristics of iCRC in this setting.
MATERIALS AND METHODS
We searched the National Cancer Screening Program for CRC database in Korea (2005-2010). Incidence of iCRC within the program was estimated, then Cox proportional-hazards regression analysis was performed to determine the independent predictors of iCRC. The clinical characteristics of iCRC were compared with screen-detected CRC (sCRC).
RESULTS
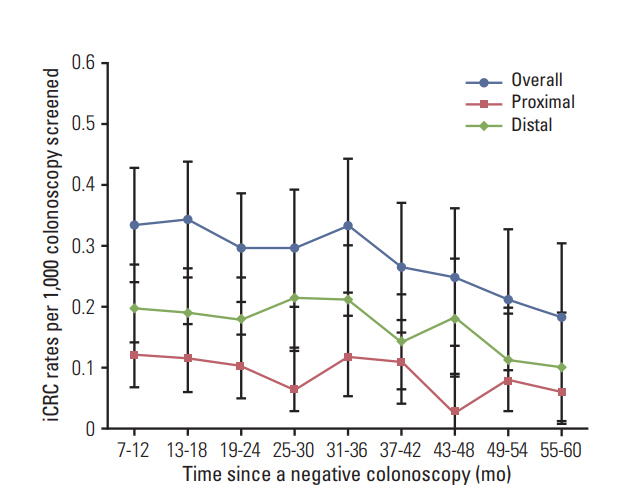
We identified 280 iCRC among 150,660 negative colonoscopies as a follow-up exam to a positive fecal immunochemical test (FIT), and 2,427 sCRC. The overall incidence of iCRC was 0.49/1,000 person-years (95% confidence interval [CI], 0.48 to 0.51). iCRC was more likely to occur in men (adjusted hazard ratio [aHR], 1.79; 95% CI, 1.39 to 2.30) and elderly patients (aHR, 1.77; 95% CI, 1.38 to 2.28 in 65-74 years; aHR, 3.13, 95% CI, 2.13 to 4.60 in ≥ 75 years). The National Quality Improvement Program for colonoscopy reduced a short-term risk of iCRC (aHR, 0.48; 95% CI, 0.27 to 0.87). Compared with sCRC, iCRC was more likely to occur in the proximal colon, be diagnosed at the localized stage, and have a lower CRC mortality (32.7 vs. 17.4%, 56.8 vs. 34.1%, and 12.5 vs. 17.7%, respectively; all p < 0.05).
CONCLUSION
In a population-based CRC screening program with FIT, the burden of iCRC after follow-up colonoscopy was substantial. Men and elderly patients possess a significantly higher risk of iCRC.
MeSH Terms
Figure
Cited by 2 articles
-
Screening strategy for colorectal cancer according to risk
Dong Soo Han
J Korean Med Assoc. 2017;60(11):893-898. doi: 10.5124/jkma.2017.60.11.893.Association between Endoscopist Volume and Interval Cancers after Colonoscopy: Results from the National Colorectal Cancer Screening Program in Korea
Dong Jun Kim, Nan-He Yoon, Jae Kwan Jun, Mina Suh, Sunhwa Lee, Seongju Kim, Ji Eun Kim, Hooyeon Lee
Cancer Res Treat. 2024;56(4):1164-1170. doi: 10.4143/crt.2024.009.
Reference
-
References
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136:E359–86.
Article2. Schreuders EH, Ruco A, Rabeneck L, Schoen RE, Sung JJ, Young GP, et al. Colorectal cancer screening: a global overview of existing programmes. Gut. 2015; 64:1637–49.
Article3. Moss S, Ancelle-Park R, Brenner H; International Agency for Research on Cancer. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First edition: evaluation and interpretation of screening outcomes. Endoscopy. 2012; 44 Suppl 3:SE49–64.4. Corley DA, Jensen CD, Marks AR, Zhao WK, Lee JK, Doubeni CA, et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014; 370:1298–306.
Article5. Kaminski MF, Regula J, Kraszewska E, Polkowski M, Wojciechowska U, Didkowska J, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010; 362:1795–803.
Article6. Singh S, Singh PP, Murad MH, Singh H, Samadder NJ. Prevalence, risk factors, and outcomes of interval colorectal cancers: a systematic review and meta-analysis. Am J Gastroenterol. 2014; 109:1375–89.
Article7. Robertson DJ, Lieberman DA, Winawer SJ, Ahnen DJ, Baron JA, Schatzkin A, et al. Colorectal cancers soon after colonoscopy: a pooled multicohort analysis. Gut. 2014; 63:949–56.
Article8. le Clercq CM, Bouwens MW, Rondagh EJ, Bakker CM, Keulen ET, de Ridder RJ, et al. Postcolonoscopy colorectal cancers are preventable: a population-based study. Gut. 2014; 63:957–63.
Article9. Cooper GS, Xu F, Barnholtz Sloan JS, Schluchter MD, Koroukian SM. Prevalence and predictors of interval colorectal cancers in medicare beneficiaries. Cancer. 2012; 118:3044–52.
Article10. Baxter NN, Sutradhar R, Forbes SS, Paszat LF, Saskin R, Rabeneck L. Analysis of administrative data finds endoscopist quality measures associated with postcolonoscopy colorectal cancer. Gastroenterology. 2011; 140:65–72.
Article11. Singh H, Nugent Z, Demers AA, Bernstein CN. Rate and predictors of early/missed colorectal cancers after colonoscopy in Manitoba: a population-based study. Am J Gastroenterol. 2010; 105:2588–96.
Article12. Singh H, Nugent Z, Mahmud SM, Demers AA, Bernstein CN. Predictors of colorectal cancer after negative colonoscopy: a population-based study. Am J Gastroenterol. 2010; 105:663–73.
Article13. Jung KW, Won YJ, Kong HJ, Oh CM, Cho H, Lee DH, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2012. Cancer Res Treat. 2015; 47:127–41.
Article14. Shim JI, Kim Y, Han MA, Lee HY, Choi KS, Jun JK, et al. Results of colorectal cancer screening of the national cancer screening program in Korea, 2008. Cancer Res Treat. 2010; 42:191–8.
Article15. Choi KS, Jun JK, Suh M, Park B, Noh DK, Song SH, et al. Effect of endoscopy screening on stage at gastric cancer diagnosis: results of the National Cancer Screening Programme in Korea. Br J Cancer. 2015; 112:608–12.
Article16. Shin A, Choi KS, Jun JK, Noh DK, Suh M, Jung KW, et al. Validity of fecal occult blood test in the national cancer screening program, Korea. PLoS One. 2013; 8:e79292.
Article17. Shin HR, Won YJ, Jung KW, Kong HJ, Yim SH, Lee JK, et al. Nationwide cancer incidence in Korea, 1999~2001; first result using the national cancer incidence database. Cancer Res Treat. 2005; 37:325–31.
Article18. Fritz A, Percy C, Jack A, Shanmugarantnam K, Sobin L, Parkin DM, et al. International classification of diseases for oncology (ICD-O). 3rd ed. Geneva: World Health Organization;2000.19. Young JL Jr, Roffers SD, Ries LA, Fritz AG, Hurlbut AA. SEER summary staging manual 2000: codes and coding instructions. Bethesda, MD: National Cancer Institute;2001.20. Pabby A, Schoen RE, Weissfeld JL, Burt R, Kikendall JW, Lance P, et al. Analysis of colorectal cancer occurrence during surveillance colonoscopy in the dietary Polyp Prevention Trial. Gastrointest Endosc. 2005; 61:385–91.
Article21. Sanduleanu S, le Clercq CM, Dekker E, Meijer GA, Rabeneck L, Rutter MD, et al. Definition and taxonomy of interval colorectal cancers: a proposal for standardising nomenclature. Gut. 2015; 64:1257–67.
Article22. Bressler B, Paszat LF, Chen Z, Rothwell DM, Vinden C, Rabeneck L. Rates of new or missed colorectal cancers after colonoscopy and their risk factors: a population-based analysis. Gastroenterology. 2007; 132:96–102.
Article23. Chiu SY, Chuang SL, Chen SL, Yen AM, Fann JC, Chang DC, et al. Faecal haemoglobin concentration influences risk prediction of interval cancers resulting from inadequate colonoscopy quality: analysis of the Taiwanese Nationwide Colorectal Cancer Screening Program. Gut. 2017; 66:293–300.
Article24. Rondagh EJ, Bouwens MW, Riedl RG, Winkens B, de Ridder R, Kaltenbach T, et al. Endoscopic appearance of proximal colorectal neoplasms and potential implications for colonoscopy in cancer prevention. Gastrointest Endosc. 2012; 75:1218–25.
Article25. Brenner H, Chang-Claude J, Seiler CM, Sturmer T, Hoffmeister M. Does a negative screening colonoscopy ever need to be repeated? Gut. 2006; 55:1145–50.
Article26. Shin A, Kim KZ, Jung KW, Park S, Won YJ, Kim J, et al. Increasing trend of colorectal cancer incidence in Korea, 1999-2009. Cancer Res Treat. 2012; 44:219–26.
Article27. Murphy G, Devesa SS, Cross AJ, Inskip PD, McGlynn KA, Cook MB. Sex disparities in colorectal cancer incidence by anatomic subsite, race and age. Int J Cancer. 2011; 128:1668–75.
Article28. Wu X, Chen VW, Martin J, Roffers S, Groves FD, Correa CN, et al. Subsite-specific colorectal cancer incidence rates and stage distributions among Asians and Pacific Islanders in the United States, 1995 to 1999. Cancer Epidemiol Biomarkers Prev. 2004; 13:1215–22.29. Allison JE, Sakoda LC, Levin TR, Tucker JP, Tekawa IS, Cuff T, et al. Screening for colorectal neoplasms with new fecal occult blood tests: update on performance characteristics. J Natl Cancer Inst. 2007; 99:1462–70.
Article30. Cha JM, Han DS, Lee HL, Kim YH, Chung IK, Kim HS, et al. Endoscopist specialty is associated with high-quality endoscopy in Korea. Yonsei Med J. 2012; 53:310–7.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Strategies for colorectal cancer screening and post-polypectomy surveillance for young adults under age 50
- A randomized controlled trial to motivate worksite fecal occult blood testing. Lee CY
- Risk of colorectal cancer in patients with positive results of fecal immunochemical test performed within 5 years since the last colonoscopy
- Population Screening for Colorectal Cancer Means Getting FIT: The Past, Present, and Future of Colorectal Cancer Screening Using the Fecal Immunochemical Test for Hemoglobin (FIT)
- A Randomized Controlled Trial To Motivate Worksite Fecal Occult Blood Testing