Cancer Res Treat.
2015 Oct;47(4):645-652. 10.4143/crt.2014.144.
Definitive Bimodality Concurrent Chemoradiotherapy in Patients with Inoperable N2-positive Stage IIIA Non-small Cell Lung Cancer
- Affiliations
-
- 1Department of Radiation Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. ahnyc@skku.edu
- 2Department of Radiation Oncology, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Division of Hematology-Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2403382
- DOI: http://doi.org/10.4143/crt.2014.144
Abstract
- PURPOSE
This study was conducted to evaluate the treatment outcomes following definitive bimodality concurrent chemoradiotherapy (CCRT) in patients with inoperable N2-positive stage IIIA (N2-IIIA) non-small cell lung cancer (NSCLC).
MATERIALS AND METHODS
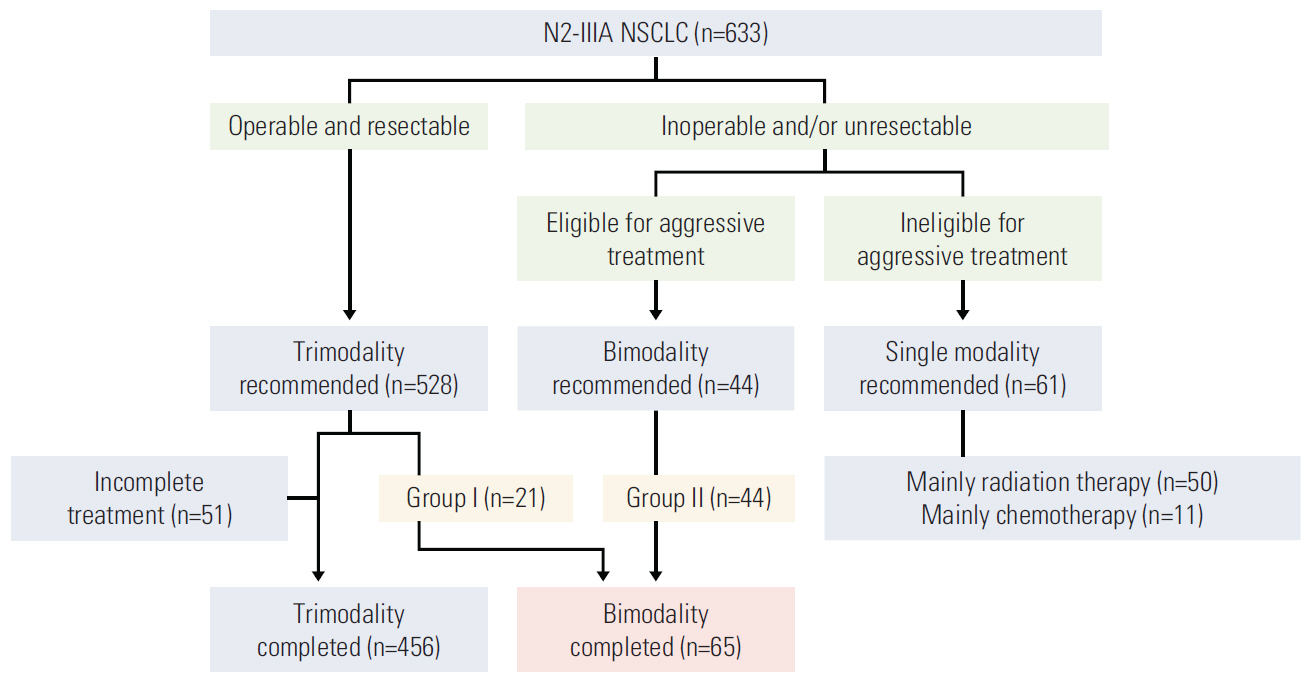
From May 1997 to December 2012, 65 out of 633 patients with N2-IIIA NSCLC received bimodality therapy. The treatment modality was selected during/after neoadjuvant CCRT in 21 patients or primarily at diagnosis in 44 through a multidisciplinary consensus meeting. The median age was 65 years (range, 36 to 76 years). Sixty patients (92.3%) had clinically evident N2 disease, while 22 (33.8%) had multi-station N2 involvement. The median radiation therapy dose was 66 Gy in 33 fractions, while the dose was elevated to 72 Gy in 13 patients who had a treatment break due to delayed decision regarding resectability. The most frequent chemotherapy regimen was weekly paclitaxel or docetaxel plus cisplatin or carboplatin (54, 83.1%).
RESULTS
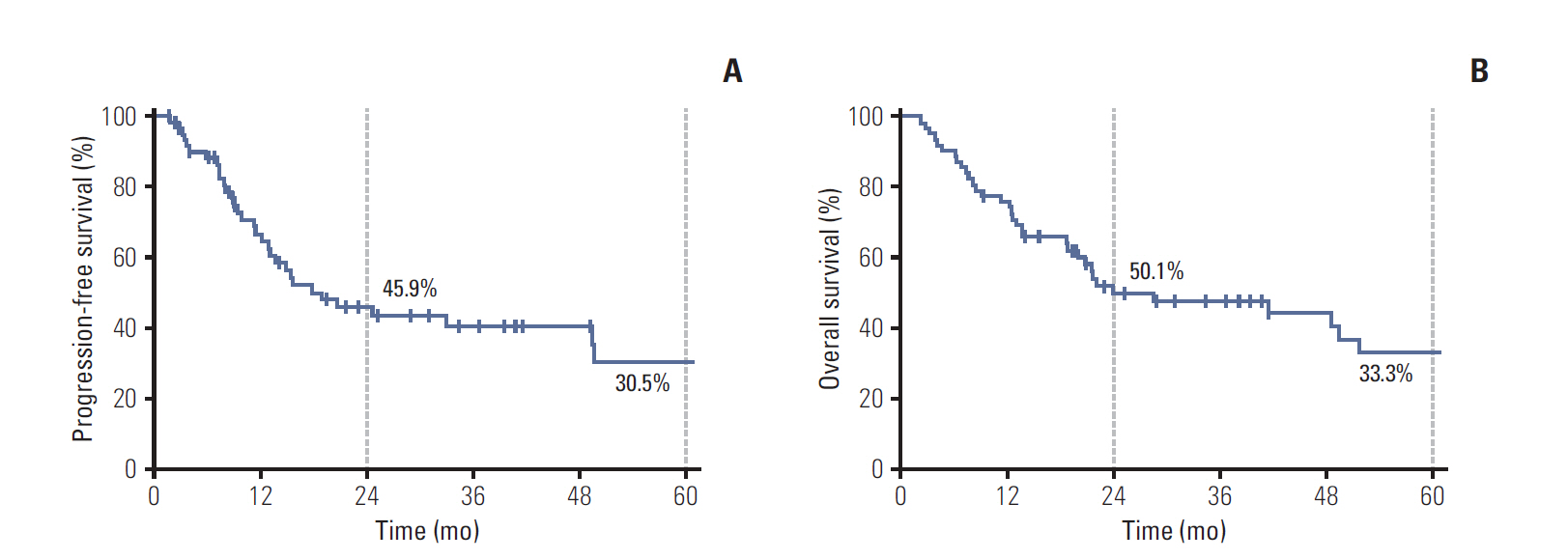
During the median follow-up of 18.8 months (range, 1.6 to 173.1 months), 34 patients (52.3%) experienced disease progression, with distant metastasis being the most common first treatment failure pattern (23, 34.8%). The median and 2-year rates of progression-free survival were 18.8 months and 45.9%, respectively. The median and 2-year rates of overall survival were 28.6 months and 50.1%, respectively.
CONCLUSION
Definitive bimodality therapy in patients with N2-IIIA NSCLC demonstrated favorable outcomes, while trimodality therapy could be considered for candidates for less than pneumonectomy.
MeSH Terms
Figure
Reference
-
References
1. Martini N, Kris MG, Ginsberg RJ. The role of multimodality therapy in locoregional non-small cell lung cancer. Surg Oncol Clin N Am. 1997; 6:769–91.
Article2. National Comprehensive Cancer Network. NCCN guidelines version 3, 2014. Non-small cell lung cancer [Internet] . Fort Washington, PA: National Comprehensive Cancer Network;[cited 2014 Jun 9] . Available from: http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.3. Ramnath N, Dilling TJ, Harris LJ, Kim AW, Michaud GC, Balekian AA, et al. Treatment of stage III non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013; 143(5 Suppl):e314S.4. Fournel P, Robinet G, Thomas P, Souquet PJ, Lena H, Vergnenegre A, et al. Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-Saint-Etienne d'Oncologie Thoracique-Groupe Francais de Pneumo-Cancerologie NPC 95-01 Study. J Clin Oncol. 2005; 23:5910–7.5. Curran WJ Jr, Paulus R, Langer CJ, Komaki R, Lee JS, Hauser S, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011; 103:1452–60.
Article6. Lee H, Ahn YC, Pyo H, Kim B, Oh D, Nam H, et al. Pretreatment clinical mediastinal nodal bulk and extent do not influence survival in N2-positive stage IIIA non-small cell lung cancer patients treated with trimodality therapy. Ann Surg Oncol. 2014; 21:2083–90.
Article7. Tanner NT, Gomez M, Rainwater C, Nietert PJ, Simon GR, Green MR, et al. Physician preferences for management of patients with stage IIIA NSCLC: impact of bulk of nodal disease on therapy selection. J Thorac Oncol. 2012; 7:365–9.
Article8. Albain KS, Swann RS, Rusch VW, Turrisi AT 3rd, Shepherd FA, Smith C, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet. 2009; 374:379–86.
Article9. Koshy M, Fedewa SA, Malik R, Ferguson MK, Vigneswaran WT, Feldman L, et al. Improved survival associated with neoadjuvant chemoradiation in patients with clinical stage IIIA(N2) non-small-cell lung cancer. J Thorac Oncol. 2013; 8:915–22.
Article10. Park J, Ahn YC, Kim H, Lee SH, Park SH, Lee KE, et al. A phase II trial of concurrent chemoradiation therapy followed by consolidation chemotherapy with oral etoposide and cisplatin for locally advanced inoperable non-small cell lung cancers. Lung Cancer. 2003; 42:227–35.
Article11. Scagliotti GV, Turrisi AT 3rd. Docetaxel-based combinedmodality chemoradiotherapy for locally advanced non-small cell lung cancer. Oncologist. 2003; 8:361–74.
Article12. Pillot G, Govindan R. The role of docetaxel in N2 locally advanced non-small-cell lung cancer. Clin Lung Cancer. 2005; 7 Suppl 3:S87–92.
Article13. Saloustros E, Georgoulias V. Docetaxel in the treatment of advanced non-small-cell lung cancer. Expert Rev Anticancer Ther. 2008; 8:1207–22.
Article14. Oh IJ, Kim KS, Kim YC, Ban HJ, Kwon YS, Kim YI, et al. A phase III concurrent chemoradiotherapy trial with cisplatin and paclitaxel or docetaxel or gemcitabine in unresectable non-small cell lung cancer: KASLC 0401. Cancer Chemother Pharmacol. 2013; 72:1247–54.
Article15. Saitoh J, Saito Y, Kazumoto T, Kudo S, Yoshida D, Ichikawa A, et al. Concurrent chemoradiotherapy followed by consolidation chemotherapy with bi-weekly docetaxel and carboplatin for stage III unresectable, non-small-cell lung cancer: clinical application of a protocol used in a previous phase II study. Int J Radiat Oncol Biol Phys. 2012; 82:1791–6.
Article16. Eroglu C, Orhan O, Unal D, Dogu GG, Karaca H, Dikilitas M, et al. Concomitant chemoradiotherapy with docetaxel and c isplatin followed by consolidation chemotherapy in locally advanced unresectable non-small cell lung cancer. Ann Thorac Med. 2013; 8:109–15.17. Park K, Ahn Y, Chen M, Cho E, Kim J, Min Y, et al. A multinational phase III randomized trial with or without consolidation chemotherapy using docetaxel and cisplatin after concurrent chemoradiation in inoperable stage III non-small cell lung cancer (CCheIN): interim analysis. J Clin Oncol. 2009; 27(15S):7538.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Early Result of Surgical Resection after Pre-Operative Concurrent chemoradiotherapy for N2-Positive Stage IIIA NSCLC
- The Prognostic Significance of Multiple Station N2 in Patients with Surgically Resected Stage IIIA N2 Non-small Cell Lung Cancer
- Definitive Radiotherapy of Non-Small Cell Lung Cancer
- Lack of the Difference in Outcome between N2 and N3 Non-Small Cell Lung Cancer Patients Treated with Concurrent Chemoradiotherapy; A Retrospective Analysis of 186 Patients
- Chemoradiotherapy for Esophageal Cancer