J Vet Sci.
2018 Jan;19(1):137-143. 10.4142/jvs.2018.19.1.137.
Magnetic resonance imaging characteristics of ischemic brain infarction over time in a canine stroke model
- Affiliations
-
- 1Ian Animal Diagnostic Center, Seoul 06014, Korea.
- 2College of Veterinary Medicine, Kyungpook National University, Daegu 41566, Korea. leekj@knu.ac.kr
- 3Royal Animal Medical Center, Seoul 02117, Korea.
- 4College of Veterinary Medicine, Chungnam National University, Daejeon 34134, Korea.
- KMID: 2402704
- DOI: http://doi.org/10.4142/jvs.2018.19.1.137
Abstract
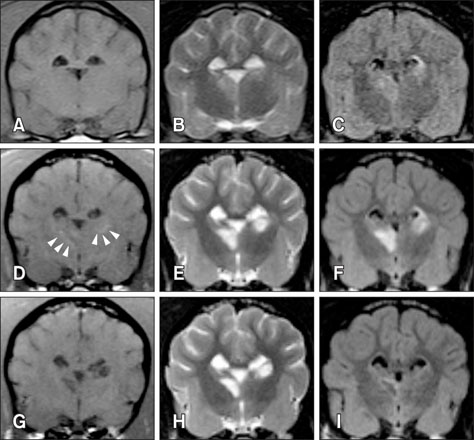
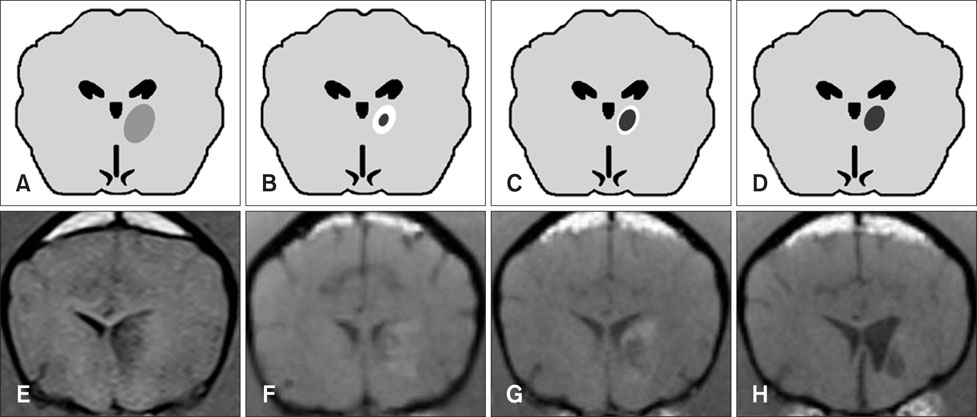
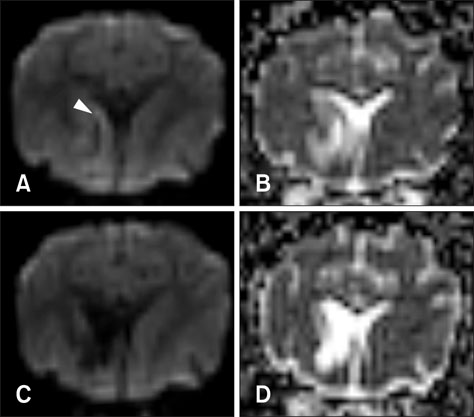
- This study describes magnetic resonance imaging (MRI) results and changes in lateral ventricular size over time in a canine ischemic stroke model. T1- and T2-weighted (T1W, T2W) imaging and fluid-attenuated inversion recovery (FLAIR) sequence MRI were performed at 3 h and 3, 8, and 35 days after brain infarct induction. Diffusion-weighted imaging (DWI) and apparent diffusion coefficient (ADC) mapping were performed at 8 and 35 days. A total of 29 brain lesions were induced successfully in 12 of 14 beagle dogs. At 3 h, T2W and FLAIR detected hyperintense lesions in three randomly selected dogs. On T1W, all lesions appeared hypointense to isointense at 3 h, isointense (18/29) or hypointense (11/29) at 3 days, hypointense to isointense with peripheral hyperintensity (24/26) at 8 days, and hypointense (18/26) at 35 days. Infarcts on DWI/ADC were hypointense to isointense centrally, with the periphery hyperintense/hyperintense (17/26) at 8 days and hypointense/hyperintense (19/26) at 35 days. A marked increase in lateral ventricular size was observed in dogs with cerebral infarcts. In conclusion, T2W and FLAIR were useful for detecting early stage (3 h to 3 days) brain infarction. T1W and DWI were useful for detecting neuronal necrosis and providing supplemental information for phase evaluation.
MeSH Terms
Figure
Reference
-
1. Allen LM, Hasso AN, Handwerker J, Farid H. Sequence-specific MR imaging findings that are useful in dating ischemic stroke. Radiographics. 2012; 32:1285–1297.
Article2. Baheti NN, Cherian A, Wattamwar PR, Kesavadas C, Thomas B. Ischemic hyperintensities on T1-weighted magnetic resonance imaging of patients with stroke: new insights from susceptibility weighted imaging. Neurol India. 2010; 58:90–94.
Article3. Bezuidenhout AJ. The heart and arteries. In : Evans HE, de Lahunta A, editors. Miller's Anatomy of the Dog. 4th ed. St. Louis: W.B Saunders;2013. p. 428–504.4. Ferrand-Drake M, Wieloch T. The time-course of DNA fragmentation in the choroid plexus and the CA1 region following transient global ischemia in the rat brain. The effect of intra-ischemic hypothermia. Neuroscience. 1999; 93:537–549.
Article5. Garosi L, McConnell JF, Platt SR, Barone G, Baron JC, de Lahunta A, Schatzberg SJ. Clinical and topographic magnetic resonance characteristics of suspected brain infarction in 40 dogs. J Vet Intern Med. 2006; 20:311–321.
Article6. Gillardon F, Lenz C, Kuschinsky W, Zimmermann M. Evidence for apoptotic cell death in the choroid plexus following focal cerebral ischemia. Neurosci Lett. 1996; 207:113–116.
Article7. Gonçalves R, Carrera I, Garosi L, Smith PM, Fraser McConnell J, Penderis J. Clinical and topographic magnetic resonance imaging characteristics of suspected thalamic infarcts in 16 dogs. Vet J. 2011; 188:39–43.
Article8. Jeon JH, Jung HW, Jang HM, Moon JH, Park KT, Lee HC, Lim HY, Sur JH, Kang BT, Ha J, Jung DI. Canine model of ischemic stroke with permanent middle cerebral artery occlusion: clinical features, magnetic resonance imaging, histopathology, and immunohistochemistry. J Vet Sci. 2015; 16:75–85.
Article9. Kang BT, Jang DP, Gu SH, Lee JH, Jung DI, Lim CY, Kim HJ, Kim YB, Kim HJ, Woo EJ, Cho ZH, Park HM. MRI features in a canine model of ischemic stroke: correlation between lesion volume and neurobehavioral status during the subacute stage. Comp Med. 2009; 59:459–464.10. Kii S, Uzuka Y, Taura Y, Nakaichi M, Takeuchi A, Inokuma H, Onishi T. Magnetic resonance imaging of the lateral ventricles in beagle-type dogs. Vet Radiol Ultrasound. 1997; 38:430–433.
Article11. Kim B, Yi K, Jung S, Ji S, Choi M, Yoon J. Clinical applications and characteristics of apparent diffusion coefficient maps for the brain of two dogs. J Vet Sci. 2014; 15:455–458.
Article12. Liu S, Hu WX, Zu QQ, Lu SS, Xu XQ, Sun L, Zhou WZ, Shi HB. A novel embolic stroke model resembling lacunar infarction following proximal middle cerebral artery occlusion in beagle dogs. J Neurosci Methods. 2012; 209:90–96.
Article13. McConnell JF, Garosi L, Platt SR. Magnetic resonance imaging findings of presumed cerebellar cerebrovascular accident in twelve dogs. Vet Radiol Ultrasound. 2005; 46:1–10.
Article14. Meyer JR, Gutierrez A, Mock B, Hebron D, Prager JM, Gorey MT, Homer D. High-b-value diffusion-weighted MR imaging of suspected brain infarction. AJNR Am J Neuroradiol. 2000; 21:1821–1829.15. Molinari GF. Experimental cerebral infarction. I. Selective segmental occlusion of intracranial arteries in the dog. Stroke. 1970; 1:224–231.
Article16. Mukherji SK, Chenevert TL, Castillo M. Diffusion-weighted magnetic resonance imaging. J Neuroophthalmol. 2002; 22:118–122.
Article17. Shook BA, Lennington JB, Acabchuk RL, Halling M, Sun Y, Peters J, Wu Q, Mahajan A, Fellows DW, Conover JC. Ventriculomegaly associated with ependymal gliosis and declines in barrier integrity in the aging human and mouse brain. Aging Cell. 2014; 13:340–350.
Article18. Srinivasan A, Goyal M, Al Azri F, Lum C. State-of-the-art imaging of acute stroke. Radiographics. 2006; 26:Suppl 1. S75–S95.
Article19. Wessmann A, Chandler K, Garosi L. Ischaemic and haemorrhagic stroke in the dog. Vet J. 2009; 180:290–303.
Article20. Winship IR. Cerebral collaterals and collateral therapeutics for acute ischemic stroke. Microcirculation. 2015; 22:228–236.
Article21. Xu XQ, Cheng QG, Zu QQ, Lu SS, Yu J, Sheng Y, Shi HB, Liu S. Comparative study of the relative signal intensity on DWI, FLAIR, and T2 images in identifying the onset time of stroke in an embolic canine model. Neurol Sci. 2014; 35:1059–1065.
Article22. Xu XQ, Zu QQ, Lu SS, Cheng QG, Yu J, Sheng Y, Shi HB, Liu S. Use of FLAIR imaging to identify onset time of cerebral ischemia in a canine model. AJNR Am J Neuroradiol. 2014; 35:311–316.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Added Prognostic Value of Intracranial Artery Morphology to Predict Non-Cardioembolic Ischemic Stroke
- Silent New Brain Lesions: Innocent Bystander or Guilty Party?
- Persistent Trigeminal Artery : An Unusual Cause of Cerebral Infarction
- Different Lesion Patterns in Cerebral Infarction Associated with Hypereosinophilic Syndrome
- Imaging of the Ischemic Penumbra in Acute Stroke