Asian Organization for Crohn's and Colitis and Asia Pacific Association of Gastroenterology consensus on tuberculosis infection in patients with inflammatory bowel disease receiving anti-tumor necrosis factor treatment. Part 1: risk assessment
- Affiliations
-
- 1Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 2The Third Department of Internal Medicine, Kyorin University School of Medicine, Tokyo, Japan.
- 3Department of Gastroenterology, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China.
- 4Department of Medicine and Therapeutics, Institute of Digestive Disease, LKS Institute of Health Science, State Key Laboratory of Digestive Disease, The Chinese University of Hong Kong, Hong Kong, China.
- 5Gleneagles Medical Centre and Duke-NUS Medical School, Singapore, Singapore.
- 6Department of Internal Medicine, National Taiwan University Hospital, National Taiwan University College of Medicine, Taipei, Taiwan.
- 7Department of Medical Gastroenterology, Asian Institute of Gastroenterology, Hyderabad, India.
- 8Department of Medicine, University of Malaya, Kuala Lumpur, Malaysia.
- 9Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea.
- 10Department of Internal Medicine, Hanyang University Guri Hospital, Guri, Korea.
- 11Department of Internal Medicine, Kyung Hee University School of Medicine, Seoul, Korea.
- 12Department of Gastroenterology, Shanghai Jiao Tong University, Shanghai, China.
- 13Department of Gastroenterology, Fourth Military Medical University, Xi'an, China.
- 14Department of Gastroenterology, Peking Union Medical College, Beijing, China.
- 15Department of Gastroenterology and Hepatology, Tokyo Medical and Dental University, Tokyo, Japan.
- 16Department of Gastroenterology, Shiga University, Otsu, Japan.
- 17Department of Internal Medicine, Toho University, Sakura, Japan.
- 18Department of Medicine, Jichi Medical University, Shimotsuke, Japan.
- 19Center for Advanced IBD Research and Treatment, Kitasato University, Tokyo, Japan.
- 20Department of Gastroenterology, Govind Ballabh Pant Institute of Postgraduate Medical Education and Research, New Delhi, India.
- 21Department of Gastroenterology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. sky@amc.seoul.kr
- KMID: 2402642
- DOI: http://doi.org/10.5217/ir.2018.16.1.4
Abstract
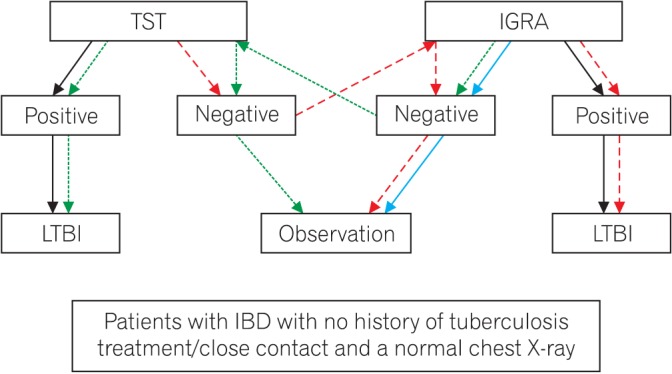
- Because anti-tumor necrosis factor (anti-TNF) therapy has become increasingly popular in many Asian countries, the risk of developing active tuberculosis (TB) among anti-TNF users may raise serious health problems in this region. Thus, the Asian Organization for Crohn's and Colitis and the Asia Pacific Association of Gastroenterology have developed a set of consensus statements about risk assessment, detection and prevention of latent TB infection, and management of active TB infection in patients with inflammatory bowel disease (IBD) receiving anti-TNF treatment. Twenty-three consensus statements were initially drafted and then discussed by the committee members. The quality of evidence and the strength of recommendations were assessed by using the Grading of Recommendations Assessment, Development, and Evaluation methodology. Web-based consensus voting was performed by 211 IBD specialists from 9 Asian countries concerning each statement. A consensus statement was accepted if at least 75% of the participants agreed. Part 1 of the statements comprised 2 parts: risk of TB infection Recommendaduring anti-TNF therapy, and screening for TB infection prior to commencing anti-TNF therapy. These consensus statements will help clinicians optimize patient outcomes by reducing the morbidity and mortality related to TB infections in patients with IBD receiving anti-TNF treatment.
MeSH Terms
Figure
Cited by 6 articles
-
Multidrug-resistant Disseminated Tuberculosis Related to Infliximab in a Patient with Ulcerative Colitis and Negative Evaluation for Latent Tuberculosis
Yu Kyung Jun, Jaeyoung Chun, Eun Ae Kang, Hyun Jung Lee, Jong Pil Im, Joo Sung Kim
Korean J Gastroenterol. 2019;74(3):168-174. doi: 10.4166/kjg.2019.74.3.168.Successful treatment with vedolizumab in an adolescent with Crohn disease who had developed active pulmonary tuberculosis while receiving infliximab
Sujin Choi, Bong Seok Choi, Byung-Ho Choe, Ben Kang
Yeungnam Univ J Med. 2021;38(3):251-257. doi: 10.12701/yujm.2020.00878.Safety and effectiveness of adalimumab in the treatment of ulcerative colitis: results from a large-scale, prospective, multicenter, observational study
Haruhiko Ogata, Takashi Hagiwara, Takeshi Kawaberi, Mariko Kobayashi, Toshifumi Hibi
Intest Res. 2021;19(4):419-429. doi: 10.5217/ir.2020.00033.Evidence-based consensus on opportunistic infections in inflammatory bowel disease (republication)
Intest Res. 2018;16(2):178-193. doi: 10.5217/ir.2018.16.2.178.High risk of tuberculosis during infliximab therapy despite tuberculosis screening in inflammatory bowel disease patients in India
Ashish Agarwal, Saurabh Kedia, Saransh Jain, Vipin Gupta, Sawan Bopanna, Dawesh P Yadav, Sandeep Goyal, Venigalla Pratap Mouli, Rajan Dhingra, Govind Makharia, Vineet Ahuja
Intest Res. 2018;16(4):588-598. doi: 10.5217/ir.2018.00023.Korean clinical practice guidelines on biologics and small molecules for moderate-to-severe ulcerative colitis
Soo-Young Na, Chang Hwan Choi, Eun Mi Song, Ki Bae Bang, Sang Hyoung Park, Eun Soo Kim, Jae Jun Park, Bora Keum, Chang Kyun Lee, Bo-In Lee, Seung-Bum Ryoo, Seong-Joon Koh, Miyoung Choi, Joo Sung Kim
Intest Res. 2023;21(1):61-87. doi: 10.5217/ir.2022.00007.
Reference
-
1. Getahun H, Matteelli A, Chaisson RE, Raviglione M. Latent Mycobacterium tuberculosis infection. N Engl J Med. 2015; 372:2127–2135. PMID: 26017823.2. Mack U, Migliori GB, Sester M, et al. LTBI: latent tuberculosis infection or lasting immune responses to M. tuberculosis? A TBNET consensus statement. Eur Respir J. 2009; 33:956–973. PMID: 19407047.
Article3. Fallahi-Sichani M, El-Kebir M, Marino S, Kirschner DE, Linderman JJ. Multiscale computational modeling reveals a critical role for TNF-alpha receptor 1 dynamics in tuberculosis granuloma formation. J Immunol. 2011; 186:3472–3483. PMID: 21321109.
Article4. Roach DR, Bean AG, Demangel C, France MP, Briscoe H, Britton WJ. TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J Immunol. 2002; 168:4620–4627. PMID: 11971010.
Article5. Mohan VP, Scanga CA, Yu K, et al. Effects of tumor necrosis factor alpha on host immune response in chronic persistent tuberculosis: possible role for limiting pathology. Infect Immun. 2001; 69:1847–1855. PMID: 11179363.
Article6. Rutgeerts P, Van Assche G, Vermeire S. Review article: infliximab therapy for inflammatory bowel disease. Seven years on. Aliment Pharmacol Ther. 2006; 23:451–463. PMID: 16441465.
Article7. Wallis RS, Broder MS, Wong JY, Hanson ME, Beenhouwer DO. Granulomatous infectious diseases associated with tumor necrosis factor antagonists. Clin Infect Dis. 2004; 38:1261–1265. PMID: 15127338.
Article8. Tubach F, Salmon D, Ravaud P, et al. Risk of tuberculosis is higher with anti-tumor necrosis factor monoclonal antibody therapy than with soluble tumor necrosis factor receptor therapy: the three-year prospective French Research Axed on Tolerance of Biotherapies registry. Arthritis Rheum. 2009; 60:1884–1894. PMID: 19565495.
Article9. Winthrop KL, Baxter R, Liu L, et al. Mycobacterial diseases and antitumour necrosis factor therapy in USA. Ann Rheum Dis. 2013; 72:37–42. PMID: 22523429.
Article10. Navarra SV, Tang B, Lu L, et al. Risk of tuberculosis with anti-tumor necrosis factor-alpha therapy: substantially higher number of patients at risk in Asia. Int J Rheum Dis. 2014; 17:291–298. PMID: 24131578.
Article11. Weng MT, Wei SC, Lin CC, et al. Seminar report from the 2014 Taiwan Society of Inflammatory Bowel Disease (TSIBD) Spring Forum (May 24th, 2014): Crohn's disease versus intestinal tuberculosis infection. Intest Res. 2015; 13:6–10. PMID: 25691838.
Article12. Song HK, Lee KM, Jung SA, et al. Quality of care in inflammatory bowel disease in Asia: the results of a multinational web-based survey in the 2(nd) Asian Organization of Crohn's and Colitis (AOCC) meeting in Seoul. Intest Res. 2016; 14:240–247. PMID: 27433146.
Article13. Carmona L, Gomez-Reino JJ, Rodriguez-Valverde V, et al. Effectiveness of recommendations to prevent reactivation of latent tuberculosis infection in patients treated with tumor necrosis factor antagonists. Arthritis Rheum. 2005; 52:1766–1772. PMID: 15934089.
Article14. Rahier JF, Ben-Horin S, Chowers Y, et al. European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2009; 3:47–91. PMID: 21172250.
Article15. Rahier JF, Magro F, Abreu C, et al. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014; 8:443–468. PMID: 24613021.
Article16. Getahun H, Matteelli A, Abubakar I, et al. Management of latent Mycobacterium tuberculosis infection: WHO guidelines for low tuberculosis burden countries. Eur Respir J. 2015; 46:1563–1576. PMID: 26405286.17. Favalli EG, Caporali R, Sinigaglia L, et al. Recommendations for the use of biologic therapy in rheumatoid arthritis: update from the Italian Society for Rheumatology II. Safety. Clin Exp Rheumatol. 2011; 29(3 Suppl 66):S15–S27.18. Prevention Committee of the Japanese Society for Tuberculosis. Treatment Committee of the Japanese Society for Tuberculosis. Treatment guidelines for latent tuberculosis infection. Kekkaku. 2014; 89:21–37. PMID: 24654427.19. Nordgaard-Lassen I, Dahlerup JF, Belard E, et al. Guidelines for screening, prophylaxis and critical information prior to initiating anti-TNF-alpha treatment. Dan Med J. 2012; 59:C4480. PMID: 22759856.20. Iannone F, Cantini F, Lapadula G. Diagnosis of latent tuberculosis and prevention of reactivation in rheumatic patients receiving biologic therapy: international recommendations. J Rheumatol Suppl. 2014; 91:41–46. PMID: 24788999.
Article21. Carpio D, Jauregui-Amezaga A, de Francisco R, et al. Tuberculo-sis in anti-tumour necrosis factor-treated inflammatory bowel disease patients after the implementation of preventive measures: compliance with recommendations and safety of retreatment. J Crohns Colitis. 2016; 10:1186–1193. PMID: 26802085.
Article22. Bombardier C, Hazlewood GS, Akhavan P, et al. Canadian Rheumatology Association recommendations for the pharmacological management of rheumatoid arthritis with traditional and biologic disease-modifying antirheumatic drugs: part II safety. J Rheumatol. 2012; 39:1583–1602. PMID: 22707613.
Article23. Shim TS. Diagnosis and treatment of latent tuberculosis infection in patients with inflammatory bowel diseases due to initiation of anti-tumor necrosis factor therapy. Intest Res. 2014; 12:12–19. PMID: 25349559.
Article24. Lorenzetti R, Zullo A, Ridola L, et al. Higher risk of tuberculosis reactivation when anti-TNF is combined with immunosuppressive agents: a systematic review of randomized controlled trials. Ann Med. 2014; 46:547–554. PMID: 25105206.
Article25. British Thoracic Society Standards of Care Committee. BTS recommendations for assessing risk and for managing Mycobacterium tuberculosis infection and disease in patients due to start anti-TNF-alpha treatment. Thorax. 2005; 60:800–805. PMID: 16055611.26. Solovic I, Sester M, Gomez-Reino JJ, et al. The risk of tuberculosis related to tumour necrosis factor antagonist therapies: a TBNET consensus statement. Eur Respir J. 2010; 36:1185–1206. PMID: 20530046.
Article27. Chebli JM, Gaburri PD, Chebli LA, et al. A guide to prepare patients with inflammatory bowel diseases for anti-TNF-alpha therapy. Med Sci Monit. 2014; 20:487–498. PMID: 24667275.
Article28. Joint Committee for the Revision of Korean Guidelines for Tuberculosis. Korean guidelines for tuberculosis. 2nd ed. Cheongju: Korean Centers for Disease Control and Prevention;2014.29. WHO handbook for guideline development. 2nd ed. Geneva: World Health Organization;2014.30. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008; 336:924–926. PMID: 18436948.
Article31. Balshem H, Helfand M, Schunemann HJ, et al. GRADE guidelines: 3. rating the quality of evidence. J Clin Epidemiol. 2011; 64:401–406. PMID: 21208779.
Article32. Guyatt GH, Oxman AD, Kunz R, et al. Going from evidence to recommendations. BMJ. 2008; 336:1049–1051. PMID: 18467413.
Article33. Lee KM, Kim YS, Seo GS, Kim TO, Yang SK. IBD Study Group of the Korean Association for the Study of Intestinal Diseases. Use of thiopurines in inflammatory bowel disease: a consensus statement by the Korean Association for the Study of Intestinal Diseases (KASID). Intest Res. 2015; 13:193–207. PMID: 26130993.34. Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001; 345:1098–1104. PMID: 11596589.
Article35. Gomez-Reino JJ, Carmona L, Valverde VR, Mola EM, Montero MD. BIOBADASER Group. Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: a multicenter active-surveillance report. Arthritis Rheum. 2003; 48:2122–2127. PMID: 12905464.
Article36. Souto A, Maneiro JR, Salgado E, Carmona L, Gomez-Reino JJ. Risk of tuberculosis in patients with chronic immune-mediated inflammatory diseases treated with biologics and tofacitinib: a systematic review and meta-analysis of randomized controlled trials and long-term extension studies. Rheumatology (Oxford). 2014; 53:1872–1885. PMID: 24821849.
Article37. Cantini F, Niccoli L, Goletti D. Adalimumab, etanercept, infliximab, and the risk of tuberculosis: data from clinical trials, national registries, and postmarketing surveillance. J Rheumatol Suppl. 2014; 91:47–55. PMID: 24789000.
Article38. Askling J, Fored CM, Brandt L, et al. Risk and case characteristics of tuberculosis in rheumatoid arthritis associated with tumor necrosis factor antagonists in Sweden. Arthritis Rheum. 2005; 52:1986–1992. PMID: 15986370.
Article39. Ford AC, Peyrin-Biroulet L. Opportunistic infections with anti-tumor necrosis factor-alpha therapy in inflammatory bowel disease: meta-analysis of randomized controlled trials. Am J Gastroenterol. 2013; 108:1268–1276. PMID: 23649185.
Article40. Jung SM, Ju JH, Park MS, et al. Risk of tuberculosis in patients treated with anti-tumor necrosis factor therapy: a nationwide study in South Korea, a country with an intermediate tuberculosis burden. Int J Rheum Dis. 2015; 18:323–330. PMID: 25557144.
Article41. Dixon WG, Hyrich KL, Watson KD, et al. Drug-specific risk of tu-berculosis in patients with rheumatoid arthritis treated with anti-TNF therapy: results from the British Society for Rheumatology Biologics Register (BSRBR). Ann Rheum Dis. 2010; 69:522–528. PMID: 19854715.
Article42. Global tuberculosis report 2013. World Health Organization Web site. Accessed July 18, 2017. http://www.who.int/tb/publications/global_report/ed/ .43. Jauregui-Amezaga A, Turon F, Ordas I, et al. Risk of developing tuberculosis under anti-TNF treatment despite latent infection screening. J Crohns Colitis. 2013; 7:208–212. PMID: 22677117.
Article44. Manosa M, Domenech E, Cabre E. Current incidence of active tuberculosis in IBD patients treated with anti-TNF agents: still room for improvement. J Crohns Colitis. 2013; 7:e499–e500. DOI: 10.1016/j.crohns.2013.04.021. PMID: 23689076.45. Kisacik B, Pamuk ON, Onat AM, et al. Characteristics predicting tuberculosis risk under tumor necrosis factor-alpha inhibitors: report from a large multicenter cohort with high background prevalence. J Rheumatol. 2016; 43:524–529. PMID: 26773107.
Article46. Kim ES, Song GA, Cho KB, et al. Significant risk and associated factors of active tuberculosis infection in Korean patients with inflammatory bowel disease using anti-TNF agents. World J Gastroenterol. 2015; 21:3308–3316. PMID: 25805938.
Article47. Byun JM, Lee CK, Rhee SY, et al. Risks for opportunistic tuberculosis infection in a cohort of 873 patients with inflammatory bowel disease receiving a tumor necrosis factor-alpha inhibitor. Scand J Gastroenterol. 2015; 50:312–320. PMID: 25581784.
Article48. Chang CW, Wei SC, Chou JW, et al. Safety and efficacy of adalimumab for patients with moderate to severe Crohn's disease: the Taiwan Society of Inflammatory Bowel Disease (TSIBD) Study. Intest Res. 2014; 12:287–292. PMID: 25374494.
Article49. Tam LS, Leung CC, Ying SK, et al. Risk of tuberculosis in patients with rheumatoid arthritis in Hong Kong: the role of TNF blockers in an area of high tuberculosis burden. Clin Exp Rheumatol. 2010; 28:679–685. PMID: 20822708.50. Deepak P, Stobaugh DJ, Ehrenpreis ED. Infectious complications of TNF-alpha inhibitor monotherapy versus combination therapy with immunomodulators in inflammatory bowel disease: analysis of the Food and Drug Administration Adverse Event Reporting System. J Gastrointestin Liver Dis. 2013; 22:269–276. PMID: 24078983.51. Abreu C, Magro F, Santos-Antunes J, et al. Tuberculosis in anti-TNF-alpha treated patients remains a problem in countries with an intermediate incidence: analysis of 25 patients matched with a control population. J Crohns Colitis. 2013; 7:e486–e492. DOI: 10.1016/j.crohns.2013.03.004. PMID: 23583099.52. Singh J, Puri AS, Sachdeva S, Sakhuja P, Arivarasan K. Rectal tuberculosis after infliximab therapy despite negative screening for latent tuberculosis in a patient with ulcerative colitis. Intest Res. 2016; 14:183–186. PMID: 27175120.
Article53. Hsia EC, Schluger N, Cush JJ, et al. Interferon-gamma release assay versus tuberculin skin test prior to treatment with golimumab, a human anti-tumor necrosis factor antibody, in patients with rheumatoid arthritis, psoriatic arthritis, or ankylosing spondylitis. Arthritis Rheum. 2012; 64:2068–2077. PMID: 22238071.
Article54. Abitbol Y, Laharie D, Cosnes J, et al. Negative screening does not rule out the risk of tuberculosis in patients with inflammatory bowel disease undergoing anti-TNF treatment: a descriptive study on the GETAID cohort. J Crohns Colitis. 2016; 10:1179–1185. PMID: 27402916.
Article55. Kim HC, Jo KW, Jung YJ, et al. Diagnosis of latent tuberculosis infection before initiation of anti-tumor necrosis factor therapy using both tuberculin skin test and QuantiFERON-TB Gold In Tube assay. Scand J Infect Dis. 2014; 46:763–769. PMID: 25195652.
Article56. Tanabe Seiyaku. Information for proper use of infliximab (in Japanese). Accessed March 29, 2017. https://ci.nii.ac.jp/naid/10025112120/ .57. Vaughn BP, Doherty GA, Gautam S, Moss AC, Cheifetz AS. Screening for tuberculosis and hepatitis B prior to the initiation of anti-tumor necrosis therapy. Inflamm Bowel Dis. 2012; 18:1057–1063. PMID: 21953829.
Article58. Ponce de Leon D, Acevedo-Vasquez E, Alvizuri S, et al. Comparison of an interferon-gamma assay with tuberculin skin testing for detection of tuberculosis (TB) infection in patients with rheumatoid arthritis in a TB-endemic population. J Rheumatol. 2008; 35:776–781. PMID: 18398944.59. Diel R, Loddenkemper R, Nienhaus A. Evidence-based comparison of commercial interferon-gamma release assays for detecting active TB: a metaanalysis. Chest. 2010; 137:952–968. PMID: 20022968.
Article60. American Thoracic Society. Centers for Disease Control and Prevention. Infectious Diseases Society of America. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: controlling tuberculosis in the United States. Am J Respir Crit Care Med. 2005; 172:1169–1227. PMID: 16249321.61. Menzies D, Pai M, Comstock G. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Ann Intern Med. 2007; 146:340–354. PMID: 17339619.
Article62. Matulis G, Juni P, Villiger PM, Gadola SD. Detection of latent tuberculosis in immunosuppressed patients with autoimmune diseases: performance of a Mycobacterium tuberculosis antigen-specific interferon gamma assay. Ann Rheum Dis. 2008; 67:84–90. PMID: 17644549.
Article63. Mazurek GH, Jereb J, Vernon A, et al. Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection: United States, 2010. MMWR Recomm Rep. 2010; 59:1–25.64. Jung YJ, Lee JY, Jo KW, et al. The ‘either test positive’ strategy for latent tuberculous infection before anti-tumour necrosis factor treatment. Int J Tuberc Lung Dis. 2014; 18:428–434. PMID: 24670697.
Article65. Canadian Agency for Drugs and Technologies in Health (CADTH). Interferon-gamma release assays testing versus tuberculosis skin testing for tuberculosis: a review of the clinical effectiveness and guidelines. Rapid response report 2011. Ottawa: CADTH;2011.66. Singanayagam A, Manalan K, Sridhar S, et al. Evaluation of screening methods for identification of patients with chronic rheumatological disease requiring tuberculosis chemoprophylaxis prior to commencement of TNF-alpha antagonist therapy. Thorax. 2013; 68:955–961. PMID: 23976779.
Article67. Reichmann MT, Marshall BG, Cummings F, Elkington PT. Tuberculosis and TNF-inhibitors: history of exposure should outweigh investigations. BMJ Case Rep. 2014; 2014:pii: bcr2013202127. DOI: 10.1136/bcr-2013-202127.
Article68. Qumseya BJ, Ananthakrishnan AN, Skaros S, et al. QuantiFERON TB gold testing for tuberculosis screening in an inflammatory bowel disease cohort in the United States. Inflamm Bowel Dis. 2011; 17:77–83. PMID: 20848501.
Article69. Diel R, Loddenkemper R, Meywald-Walter K, Niemann S, Nienhaus A. Predictive value of a whole blood IFN-gamma assay for the development of active tuberculosis disease after recent infection with Mycobacterium tuberculosis. Am J Respir Crit Care Med. 2008; 177:1164–1170. PMID: 18276940.
Article70. Bonfiglioli KR, Ribeiro AC, Moraes JC, et al. LTBI screening in rheumatoid arthritis patients prior to anti-TNF treatment in an endemic area. Int J Tuberc Lung Dis. 2014; 18:905–911. PMID: 25199003.
Article71. Singh JA, Furst DE, Bharat A, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012; 64:625–639. PMID: 22473917.
Article72. Grzybowski S, Fishaut H, Rowe J, Brown A. Tuberculosis among patients with various radiologic abnormalities, followed by the chest clinic service. Am Rev Respir Dis. 1971; 104:605–608. PMID: 5094062.73. Efficacy of various durations of isoniazid preventive therapy for tuberculosis: five years of follow-up in the IUAT trial. International Union against Tuberculosis Committee on Prophylaxis. Bull World Health Organ. 1982; 60:555–564. PMID: 6754120.74. Targeted tuberculin testing and treatment of latent tuberculosis infection. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. This is a Joint Statement of the American Thoracic Society (ATS) and the Centers for Disease Control and Prevention (CDC). This statement was endorsed by the Council of the Infectious Diseases Society of America. (IDSA), September 1999, and the sections of this statement. Am J Respir Crit Care Med. 2000; 161:S221–S247. PMID: 10764341.75. Horsburgh CR Jr. Priorities for the treatment of latent tuberculosis infection in the United States. N Engl J Med. 2004; 350:2060–2067. PMID: 15141044.
Article76. Araujo Z, de Waard JH, de Larrea CF, Borges R, Convit J. The effect of bacille Calmette-Guerin vaccine on tuberculin reactivity in indigenous children from communities with high prevalence of tuberculosis. Vaccine. 2008; 26:5575–5581. PMID: 18723065.
Article77. Chan PC, Chang LY, Wu YC, et al. Age-specific cut-offs for the tuberculin skin test to detect latent tuberculosis in BCG-vaccinated children. Int J Tuberc Lung Dis. 2008; 12:1401–1406. PMID: 19017449.78. Diel R, Loddenkemper R, Niemann S, Meywald-Walter K, Nienhaus A. Negative and positive predictive value of a whole-blood interferon-gamma release assay for developing active tuberculosis: an update. Am J Respir Crit Care Med. 2011; 183:88–95. PMID: 20802162.
Article79. Shahidi N, Fu YT, Qian H, Bressler B. Performance of interferon-gamma release assays in patients with inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis. 2012; 18:2034–2042. PMID: 22294550.
Article80. Mow WS, Abreu-Martin MT, Papadakis KA, Pitchon HE, Targan SR, Vasiliauskas EA. High incidence of anergy in inflammatory bowel disease patients limits the usefulness of PPD screening before infliximab therapy. Clin Gastroenterol Hepatol. 2004; 2:309–313. PMID: 15067625.
Article81. Schoepfer AM, Flogerzi B, Fallegger S, et al. Comparison of interferon-gamma release assay versus tuberculin skin test for tuberculosis screening in inflammatory bowel disease. Am J Gastroenterol. 2008; 103:2799–2806. PMID: 18684188.
Article82. Jasmer RM, Nahid P, Hopewell PC. Clinical practice: latent tuberculosis infection. N Engl J Med. 2002; 347:1860–1866. PMID: 12466511.83. Pai M, Riley LW, Colford JM Jr. Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect Dis. 2004; 4:761–776. PMID: 15567126.
Article84. Papay P, Eser A, Winkler S, et al. Factors impacting the results of interferon-gamma release assay and tuberculin skin test in routine screening for latent tuberculosis in patients with inflammatory bowel diseases. Inflamm Bowel Dis. 2011; 17:84–90. PMID: 20722065.
Article85. Ferrara G, Losi M, Meacci M, et al. Routine hospital use of a new commercial whole blood interferon-gamma assay for the diagnosis of tuberculosis infection. Am J Respir Crit Care Med. 2005; 172:631–635. PMID: 15961696.
Article86. Wong SH, Ip M, Tang W, et al. Performance of interferon-gamma release assay for tuberculosis screening in inflammatory bowel disease patients. Inflamm Bowel Dis. 2014; 20:2067–2072. PMID: 25159454.
Article87. Sichletidis L, Settas L, Spyratos D, Chloros D, Patakas D. Tuberculosis in patients receiving anti-TNF agents despite chemoprophylaxis. Int J Tuberc Lung Dis. 2006; 10:1127–1132. PMID: 17044206.88. Cotter J, Rosa B. The importance of IGRA in patients candidates for biological therapy. J Crohns Colitis. 2013; 7:928–929. PMID: 23507423.89. Duarte R, Campainha S, Cotter J, et al. Position paper on tuberculosis screening in patients with immune mediated inflamma-tory diseases candidates for biological therapy. Acta Reumatol Port. 2012; 37:253–259. PMID: 23348114.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Asian Organization for Crohn's and Colitis and Asia Pacific Association of Gastroenterology consensus on tuberculosis infection in patients with inflammatory bowel disease receiving anti-tumor necrosis factor treatment. Part 2: management
- Rectal tuberculosis after infliximab therapy despite negative screening for latent tuberculosis in a patient with ulcerative colitis
- Treatment of inflammatory bowel disease in Asia: the results of a multinational web-based survey in the 2nd Asian Organization of Crohn's and Colitis (AOCC) meeting in Seoul
- Evidence-based consensus on opportunistic infections in inflammatory bowel disease (republication)
- Anti-tumor Necrosis Factor Agents and Tuberculosis in Inflammatory Bowel Disease