Anat Cell Biol.
2017 Dec;50(4):301-305. 10.5115/acb.2017.50.4.301.
Application of stereological methods for unbiased estimation of sperm morphology in the mice induced by busulfan
- Affiliations
-
- 1Department of Biology and Anatomical Sciences, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran. realmastery@hotmail.com
- 2Department of Biology and Anatomical Sciences, School of Medicine, Azad University of Medical Sciences, Tehran, Iran.
- KMID: 2399899
- DOI: http://doi.org/10.5115/acb.2017.50.4.301
Abstract
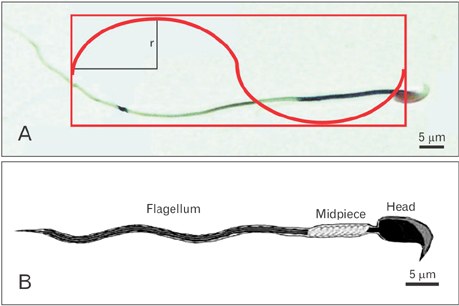
- Busulfan is an anticancer drug, which causes the apoptosis germ cells and azoospermia in humans and animals. Abnormal morphology of spermatozoa related to the male infertility. The sperm morphology is evaluation of sperm size, shape and appearance characteristics should be assessed by carefully observing a stained sperm sample under the microscope. Evaluation of sperm morphology has been considered as one of the most important factors for a successful fertilization and determining sperm quality. The mice were assigned to tow experimental groups: control and busulfan. Each group included six mice that were housed under standard conditions. The volume was estimated using the nucleator method. The sperm's flegellum and mid-piece length was estimated by counting the number of intersections between the tails and Merz grid test line in an unbiased counting frame, superimposed on live images of sperms. Our results demonstrated a significant different in the volume and surface of the sperm's head and the length of the sperm's flagellum in the control and busulfan groups. Busulfan can effect on the volume of the sperm's head and the length of the sperm's flagellum in rat.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Genistein improve nicotine toxicity on male mice pancreas
Mohammad Reza Salahshoor, Fatemeh Mirzaei, Shiva Roshankhah, Parnian Jalili, Cyrus Jalili
Anat Cell Biol. 2019;52(2):183-190. doi: 10.5115/acb.2019.52.2.183.
Reference
-
1. Howell SJ, Shalet SM. Spermatogenesis after cancer treatment: damage and recovery. J Natl Cancer Inst Monogr. 2005; (34):12–17.2. Bucci LR, Meistrich ML. Effects of busulfan on murine spermatogenesis: cytotoxicity, sterility, sperm abnormalities, and dominant lethal mutations. Mutat Res. 1987; 176:259–268.3. Iwamoto T, Hiraku Y, Oikawa S, Mizutani H, Kojima M, Kawanishi S. DNA intrastrand cross-link at the 5′-GA-3′ sequence formed by busulfan and its role in the cytotoxic effect. Cancer Sci. 2004; 95:454–458.4. Choi YJ, Ok DW, Kwon DN, Chung JI, Kim HC, Yeo SM, Kim T, Seo HG, Kim JH. Murine male germ cell apoptosis induced by busulfan treatment correlates with loss of c-kit-expression in a Fas/FasL- and p53-independent manner. FEBS Lett. 2004; 575:41–51.5. Petersen PM, Skakkebaek NE, Giwercman A. Gonadal function in men with testicular cancer: biological and clinical aspects. APMIS. 1998; 106:24–34.6. Schrader M, Müller M, Straub B, Miller K. The impact of chemotherapy on male fertility: a survey of the biologic basis and clinical aspects. Reprod Toxicol. 2001; 15:611–617.7. Chemes HE, Alvarez Sedo C. Tales of the tail and sperm head aches: changing concepts on the prognostic significance of sperm pathologies affecting the head, neck and tail. Asian J Androl. 2012; 14:14–23.8. Mosher WD. Fecundity and infertility in the United States. Am J Public Health. 1988; 78:181–182.9. Martí JI, Aparicio MI, Leal CL, García-Herreros M. Seasonal dynamics of sperm morphometric subpopulations and its association with sperm quality parameters in ram ejaculates. Theriogenology. 2012; 78:528–541.10. Rønn LC, Ralets I, Hartz BP, Bech M, Berezin A, Berezin V, Møller A, Bock E. A simple procedure for quantification of neurite outgrowth based on stereological principles. J Neurosci Methods. 2000; 100:25–32.11. Howard CV, Reed M. Unbiased stereology: three-dimensional measurement in microscopy. 2nd ed. Oxford: Bios Scientific Publisher;2005. p. 183–190.12. Kuster CE, Singer RS, Althouse GC. Determining sample size for the morphological assessment of sperm. Theriogenology. 2004; 61:691–703.13. Mossman JA, Pearson JT, Moore HD, Pacey AA. Variation in mean human sperm length is linked with semen characteristics. Hum Reprod. 2013; 28:22–32.14. de Paz P, Mata-Campuzano M, Tizado EJ, Alvarez M, Alvarez-Rodríguez M, Herraez P, Anel L. The relationship between ram sperm head morphometry and fertility depends on the procedures of acquisition and analysis used. Theriogenology. 2011; 76:1313–1325.15. Maroto-Morales A, Ramón M, García-Alvarez O, Soler AJ, Esteso MC, Martínez-Pastor F, Pérez-Guzmán MD, Garde JJ. Characterization of ram (Ovis aries) sperm head morphometry using the Sperm-Class Analyzer. Theriogenology. 2010; 73:437–448.16. Noorafshan A, Karbalay-Doust S. A simple method for unbiased estimating of ejaculated sperm tail length in subjects with normal and abnormal sperm motility. Micron. 2010; 41:96–99.17. Seed J, Chapin RE, Clegg ED, Dostal LA, Foote RH, Hurtt ME, Klinefelter GR, Makris SL, Perreault SD, Schrader S, Seyler D, Sprando R, Treinen KA, Veeramachaneni DN, Wise LD. Methods for assessing sperm motility, morphology, and counts in the rat, rabbit, and dog: a consensus report. ILSI Risk Science Institute Expert Working Group on Sperm Evaluation. Reprod Toxicol. 1996; 10:237–244.18. Gundersen HJ, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, Møller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sørensen FB, Vesterby A, West MJ. The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS. 1988; 96:857–881.19. Karlsson LM, Cruz-Orive LM. Estimation of mean particle size from single sections. J Microsc. 1997; 186:121–132.20. de Rooij DG, Vergouwen RP. The estimation of damage to testicular cell lineages. Prog Clin Biol Res. 1991; 372:467–480.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of N-acetyl-L-cysteine on Testicular Tissue in Busulfan-Induced Dysfunction in the Male Reproductive System
- Effects of Perilla frutescens Var. Acuta in Busulfan-Induced Spermatogenesis Dysfunction Mouse Model
- Stereological study of testes following experimentally-induced unilateral cryptorchidism in rats
- Effect of high-fat diet on the various morphological parameters of the ovary
- Transplantation of human umbilical cord blood CD34⺠cells into the liver of newborn NOD/SCID/IL-2Rγ null (NSG) mice after busulfan conditioning