J Clin Neurol.
2018 Jan;14(1):58-65. 10.3988/jcn.2018.14.1.58.
Clinical and Pathologic Findings of Korean Patients with RYR1-Related Congenital Myopathy
- Affiliations
-
- 1Department of Neurology, Yonsei University College of Medicine, Seoul, Korea. ycchoi@yuhs.ac
- 2Department of Neurology, Mokdong Hospital, Ewha Womans University School of Medicine, Seoul, Korea.
- 3Department of Pathology, Yonsei University College of Medicine, Seoul, Korea.
- 4Rehabilitation Institute of Neuromuscular Disease, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2399600
- DOI: http://doi.org/10.3988/jcn.2018.14.1.58
Abstract
- BACKGROUND AND PURPOSE
This study was designed to investigate clinical and pathologic characteristics of five Korean patients with RYR1-related congenital myopathy (CM).
METHODS
Five patients from unrelated families were diagnosed with RYR1-related CM via direct or targeted sequencing of RYR1. Their clinical, mutational, and pathologic findings were then analyzed.
RESULTS
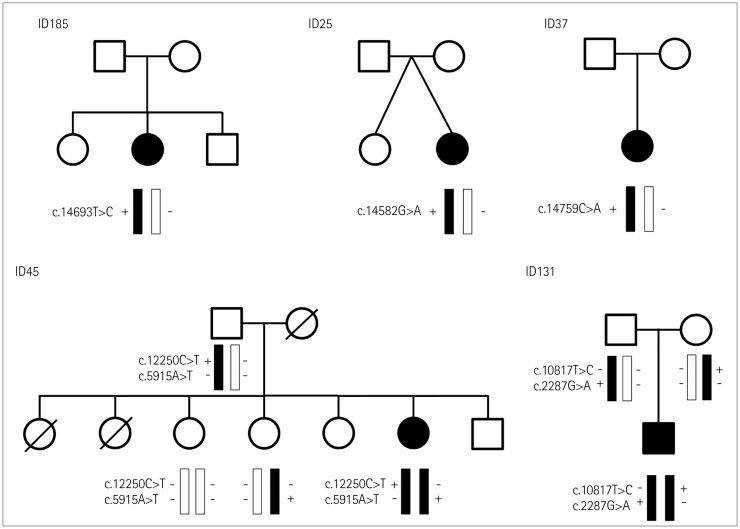
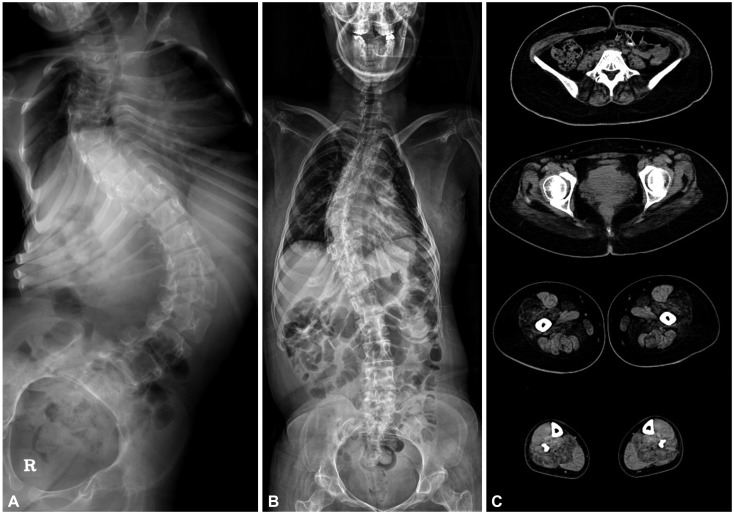
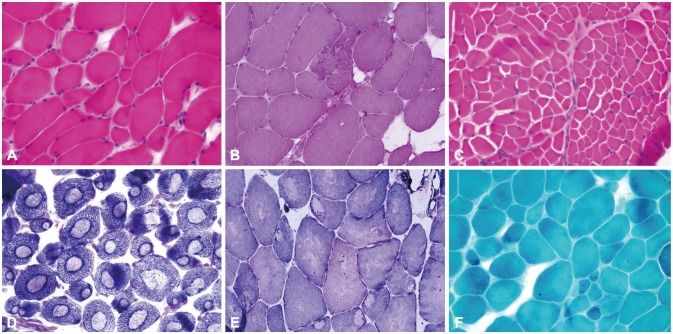
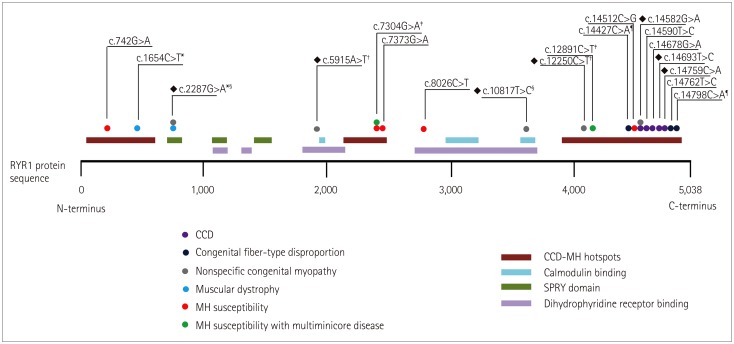
Seven different mutations were identified, including two novel mutations: c.5915A>T and c.12250C>T. All of the patients presented at infancy with proximal dominant weakness and delayed motor milestones. Other clinical findings were scoliosis in three patients, winged scapula in two, hip dislocation in one, and pectus excavatum in one. Ophthalmoplegia was observed in one patient with a novel recessive mutation. Two of three muscle specimens revealed a myopathic pattern with core.
CONCLUSIONS
We have identified a novel compound heterozygous RYR1 mutation and demonstrated clinical and pathologic findings in five Korean patients with RYR1-related CM.
Keyword
MeSH Terms
Figure
Reference
-
1. Sewry CA, Jimenez-Mallebrera C, Muntoni F. Congenital myopathies. Curr Opin Neurol. 2008; 21:569–575. PMID: 18769251.
Article2. Choi YC. Congenital dystrophies and myopathies. In : Lisak RP, Truong D, Carroll WM, Bhidayasiri R, editors. International Neurology. 2nd ed. Hoboken (NJ): John Wiley & Sons;2016. p. 485–489. .3. North KN, Wang CH, Clarke N, Jungbluth H, Vainzof M, Dowling JJ, et al. Approach to the diagnosis of congenital myopathies. Neuromuscul Disord. 2014; 24:97–116. PMID: 24456932.
Article4. Kaplan JC, Hamroun D. The 2016 version of the gene table of monogenic neuromuscular disorders (nuclear genome). Neuromuscul Disord. 2015; 25:991–1020. PMID: 27563712.
Article5. Maggi L, Scoto M, Cirak S, Robb SA, Klein A, Lillis S, et al. Congenital myopathies--clinical features and frequency of individual subtypes diagnosed over a 5-year period in the United Kingdom. Neuromuscul Disord. 2013; 23:195–205. PMID: 23394784.6. Colombo I, Scoto M, Manzur AY, Robb SA, Maggi L, Gowda V, et al. Congenital myopathies: natural history of a large pediatric cohort. Neurology. 2015; 84:28–35. PMID: 25428687.
Article7. Amburgey K, McNamara N, Bennett LR, McCormick ME, Acsadi G, Dowling JJ. Prevalence of congenital myopathies in a representative pediatric united states population. Ann Neurol. 2011; 70:662–665. PMID: 22028225.
Article8. Norwood FL, Harling C, Chinnery PF, Eagle M, Bushby K, Straub V. Prevalence of genetic muscle disease in Northern England: in-depth analysis of a muscle clinic population. Brain. 2009; 132:3175–3186. PMID: 19767415.
Article9. Amburgey K, Bailey A, Hwang JH, Tarnopolsky MA, Bonnemann CG, Medne L, et al. Genotype-phenotype correlations in recessive RYR1-related myopathies. Orphanet J Rare Dis. 2013; 8:117. PMID: 23919265.
Article10. Park HJ, Jang H, Kim JH, Lee JH, Shin HY, Kim SM, et al. Discovery of pathogenic variants in a large Korean cohort of inherited muscular disorders. Clin Genet. 2017; 91:403–410. PMID: 27363342.
Article11. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015; 17:405–424. PMID: 25741868.
Article12. Wu S, Ibarra MC, Malicdan MC, Murayama K, Ichihara Y, Kikuchi H, et al. Central core disease is due to RYR1 mutations in more than 90% of patients. Brain. 2006; 129:1470–1480. PMID: 16621918.
Article13. Broman M, Islander G, Müller CR, Ranklev-Twetman E. Malignant hyperthermia and central core disease causative mutations in Swedish patients. Acta Anaesthesiol Scand. 2007; 51:50–53. PMID: 17081152.
Article14. Chae JH, Vasta V, Cho A, Lim BC, Zhang Q, Eun SH, et al. Utility of next generation sequencing in genetic diagnosis of early onset neuromuscular disorders. J Med Genet. 2015; 52:208–216. PMID: 25635128.
Article15. Klein A, Jungbluth H, Clement E, Lillis S, Abbs S, Munot P, et al. Muscle magnetic resonance imaging in congenital myopathies due to ryanodine receptor type 1 gene mutations. Arch Neurol. 2011; 68:1171–1179. PMID: 21911697.
Article16. Jeong SK, Kim DC, Cho YG, Sunwoo IN, Kim DS. A double mutation of the ryanodine receptor type 1 gene in a malignant hyperthermia family with multiminicore myopathy. J Clin Neurol. 2008; 4:123–130. PMID: 19513315.
Article17. Lee H, Kim DC, Lee JH, Cho YG, Lee HS, Choi SI, et al. [Molecular genetic analysis of the ryanodine receptor gene (RYR1) in Korean malignant hyperthermia families]. Korean J Lab Med. 2010; 30:702–710. PMID: 21157159.
Article18. Lee JS, Lim BC, Kim KJ, Hwang YS, Seong MW, Park SS, et al. Rare coincidence of familial central core disease and hemophagocytic lymphohistiocytosis. Pediatr Int. 2014; 56:e88–e91. PMID: 25521991.
Article19. Jung NY, Park YE, Shin JH, Lee CH, Jung DS, Kim DS. Mild clinical features and histopathologically atypical cores in two Korean families with central core disease harboring RYR1 mutations at the C-terminal region. J Clin Neurol. 2015; 11:97–101. PMID: 25628744.20. Kim DC, Kim DS. Identification of G7304A mutation in the ryanodine receptor type 1 gene in a patient with malignant hyperthermia and an extended pedigree study in a Korean malignant hyperthermia family. Korean J Anesthesiol. 2003; 44:56–64.
Article21. Monnier N, Romero NB, Lerale J, Landrieu P, Nivoche Y, Fardeau M, et al. Familial and sporadic forms of central core disease are associated with mutations in the C-terminal domain of the skeletal muscle ryanodine receptor. Hum Mol Genet. 2001; 10:2581–2592. PMID: 11709545.
Article22. Snoeck M, van Engelen BG, Küsters B, Lammens M, Meijer R, Molenaar JP, et al. RYR1-related myopathies: a wide spectrum of phenotypes throughout life. Eur J Neurol. 2015; 22:1094–1112. PMID: 25960145.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mild Clinical Features and Histopathologically Atypical Cores in Two Korean Families with Central Core Disease Harboring RYR1 Mutations at the C-Terminal Region
- A Double Mutation of the Ryanodine Receptor Type 1 Gene in a Malignant Hyperthermia Family with Multiminicore Myopathy
- A Family of Congenital Fiber Type Disproportion with Mutation in Tropomyosin 3 (TPM3) Gene Presenting as Altered Mentality with Respiratory Distress
- A Case of Centronuclear Myopathy
- Myotubular myopathy: A case report