J Clin Neurol.
2008 Sep;4(3):123-130. 10.3988/jcn.2008.4.3.123.
A Double Mutation of the Ryanodine Receptor Type 1 Gene in a Malignant Hyperthermia Family with Multiminicore Myopathy
- Affiliations
-
- 1Department of Neurology, Chonbuk National University Medical School, Jeonju, Korea.
- 2Department of Anesthesiology and Pain Medicine, Chonbuk National University Medical School, Jeonju, Korea.
- 3Department of Laboratory Medicine, Chonbuk National University Medical School, Jeonju, Korea. dskim@chonbuk.ac.kr
- 4Department of Neurology, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2287676
- DOI: http://doi.org/10.3988/jcn.2008.4.3.123
Abstract
-
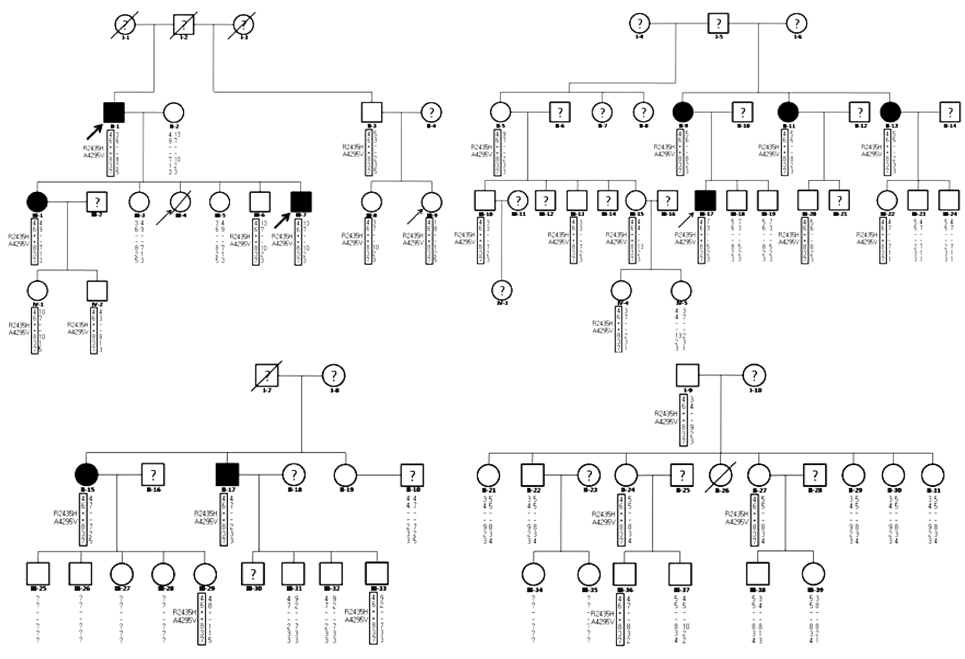
BACKGROUND AND PURPOSE: At least 100 Ryanodine receptor type 1 (RYR1) mutations associated with malignant hyperthermia (MH) and central core disease (CCD) have been identified, but 2 RYR1 mutations accompanying multiminicore myopathy in an MH and/or CCD family have been reported only rarely.
METHODS
Fifty-three members of a large MH family were investigated with clinical, histopathologic, RYR1 mutation, and haplotyping studies. Blood creatine kinase (CK) and myoglobin levels were also measured where possible.
RESULTS
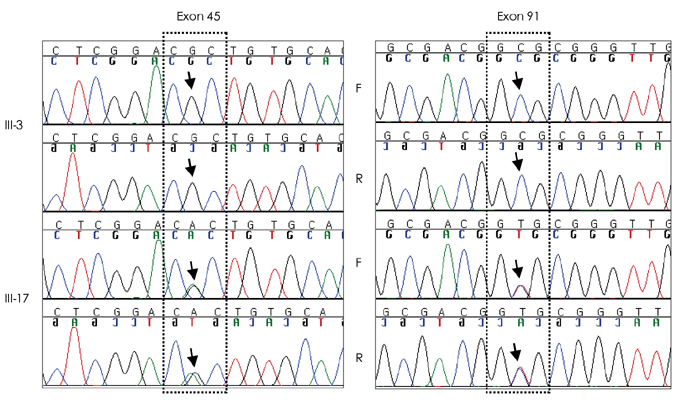
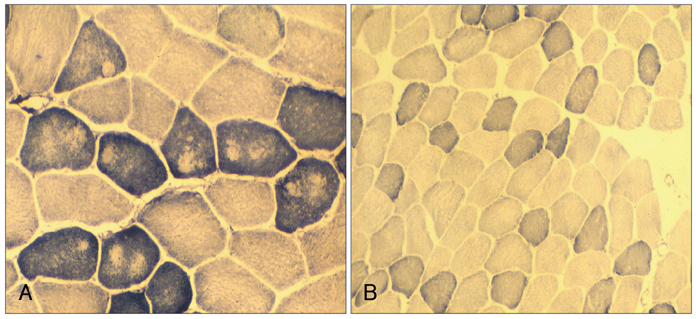
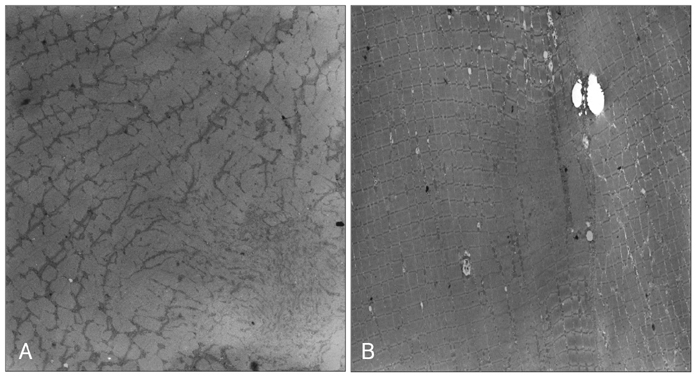
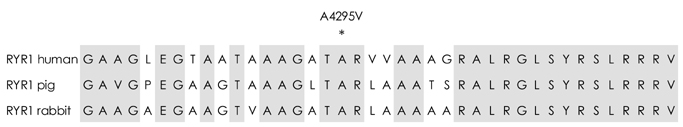
Sequencing of the entire RYR1 coding region identified a double RYR1 mutation (R2435H and A4295V) in MH/CCD regions 2 and 3. Haplotyping analysis revealed that the two missense heterozygous mutations (c.7304G>A and c.12891C>T) were always present on a common haplotype allele, and were closely cosegregated with histological multiminicores and elevated serum CK. All the subjects with the double mutation showed elevated serum CK and myoglobin, and the obtained muscle biopsy samples showed multiminicore lesions, but only two family members presented a late-onset, slowly progressive myopathy.
CONCLUSIONS
We found multiminicore myopathy with clinical and histological variability in a large MH family with an unusual double RYR1 mutation, including a typical CCD-causing known mutant. These results suggest that multiminicore lesions are associated with the presence of more than two mutations in the RYR1 gene.
MeSH Terms
-
Alleles
Biopsy
Clinical Coding
Creatine Kinase
Haplotypes
Humans
Malignant Hyperthermia
Muscles
Muscular Diseases
Myoglobin
Myopathies, Structural, Congenital
Myopathy, Central Core
Ophthalmoplegia
Ryanodine
Ryanodine Receptor Calcium Release Channel
Creatine Kinase
Myoglobin
Myopathies, Structural, Congenital
Ophthalmoplegia
Ryanodine
Ryanodine Receptor Calcium Release Channel
Figure
Cited by 2 articles
-
Clinical and Pathologic Findings of Korean Patients with
RYR1 -Related Congenital Myopathy
Ha-Neul Jeong, Hyung Jun Park, Jung Hwan Lee, Ha Young Shin, Se Hoon Kim, Seung Min Kim, Young-Chul Choi
J Clin Neurol. 2018;14(1):58-65. doi: 10.3988/jcn.2018.14.1.58.Malignant hyperthermia
Dong-Chan Kim
Korean J Anesthesiol. 2012;63(5):391-401. doi: 10.4097/kjae.2012.63.5.391.
Reference
-
1. Denborough M. Malignant hyperthermia. Lancet. 1998. 352:1131–1136.
Article2. Hopkins PM. Malignant hyperthermia: advances in clinical management and diagnosis. Br J Anaesth. 2000. 85:118–128.
Article3. Quinlivan RM, Muller CR, Davis M, Laing NG, Evans GA, Dwyer J, et al. Central core disease: clinical, pathological, and genetic features. Arch Dis Child. 2003. 88:1051–1055.
Article4. Sewry CA, Müller C, Davis M, Dwyer JS, Dove J, Evans G, et al. The spectrum of pathology in central core disease. Neuromuscul Disord. 2002. 12:930–938.
Article5. Mertz KD, Jost B, Glatzel M, Min K. Progressive scoliosis in central core disease. Eur Spine J. 2005. 14:900–905.
Article6. Jurkat-Rott K, McCarthy T, Lehmann-Horn F. Genetics and pathogenesis of malignant hyperthermia. Muscle Nerve. 2000. 23:4–17.
Article7. Robinson RL, Curran JL, Ellis FR, Halsall PJ, Hall WJ, Hopkins PM, et al. Multiple interacting gene products may influence susceptibility to malignant hyperthermia. Ann Hum Genet. 2000. 64:307–320.
Article8. MacLennan DH, Duff C, Zorzato F, Fujii J, Phillips M, Korneluk RG, et al. Ryanodine receptor gene is a candidate for predisposition to malignant hyperthermia. Nature. 1990. 343:559–561.
Article9. McCarthy TV, Healy JM, Heffron JJ, Lehane M, Deufel T, Lehmann-Horn F, et al. Localization of the malignant hyperthermia susceptibility locus to human chromosome 19q12-13.2. Nature. 1990. 343:562–564.
Article10. Kausch K, Lehmann-Horn F, Janka M, Wieringa B, Grimm T, Müller CR. Evidence for linkage of the central core disease locus to the proximal long arm of human chromosome 19. Genomics. 1991. 10:765–769.
Article11. Takeshima H, Nishimura S, Matsumoto T, Ishida H, Kangawa K, Minamino N, et al. Primary structure and expression from complementary DNA of skeletal muscle ryanodine receptor. Nature. 1989. 339:439–445.
Article12. Zorzato F, Fujii J, Otsu K, Phillips M, Green NM, Lai FA, et al. Molecular cloning of cDNA encoding human and rabbit forms of the Ca2+ release channel (ryanodine receptor) of skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1990. 265:2244–2256.
Article13. Phillips MS, Fujii J, Khanna VK, DeLeon S, Yokobata K, de Jong PJ, et al. The structural organization of the human skeletal muscle ryanodine receptor (RYR1) gene. Genomics. 1996. 34:24–41.
Article14. Brini M, Manni S, Pierobon N, Du GG, Sharma P, MacLennan DH, et al. Ca2+ signaling in HEK-293 and skeletal muscle cells expressing recombinant ryanodine receptors harboring malignant hyperthermia and central core disease mutations. J Biol Chem. 2005. 280:15380–15389.
Article15. Sambuughin N, Holley H, Muldoon S, Brandom BW, de Bantel AM, Tobin JR, et al. Screening of the entire ryanodine receptor type 1 coding region for sequence variants associated with malignant hyperthermia susceptibility in the north american population. Anesthesiology. 2005. 102:515–521.
Article16. Brandom BW, Muldoon SM. XXIVth Annual Meeting of the European Malignant Hyperthermia Group. Anesthesiology. 2005. 103:1324.
Article17. Larach MG, Localio AR, Allen GC, Denborough MA, Ellis FR, Gronert GA, et al. A clinical grading scale to predict malignant hyperthermia susceptibility. Anesthesiology. 1994. 80:771–779.
Article18. Kim JS, Moon JI, Lee JH. Malignant hyperthermia during general anesthesia (a case report). Korean J Anesthesiol. 1981. 14:313–318.
Article19. Higasa K, Hayashi K. Ordered catenation of sequence-tagged sites and multiplexed SNP genotyping by sequencing. Nucleic Acids Res. 2002. 30:E11.
Article20. Zhang Y, Chen HS, Khanna VK, De Leon S, Phillips MS, Schappert K, et al. A mutation in the human ryanodine receptor gene associated with central core disease. Nat Genet. 1993. 5:46–50.
Article21. Monnier N, Krivosic-Horber R, Payen JF, Kozak-Ribbens G, Nivoche Y, Adnet P, et al. Presence of two different genetic traits in malignant hyperthermia families: implication for genetic analysis, diagnosis, and incidence of malignant hyperthermia susceptibility. Anesthesiology. 2002. 97:1067–1074.
Article22. Romero NB, Nivoche Y, Lunardi J, Bruneau B, Cheval MA, Hillaire D, et al. Malignant hyperthermia and central core disease: analysis of two families with heterogeneous clinical expression. Neuromuscul Disord. 1993. 3:547–551.
Article23. Urwyler A, Deufel T, McCarthy T, West S; European Malignant Hyperthermia Group. Guidelines for molecular genetic detection of susceptibility to malignant hyperthermia. Br J Anaesth. 2001. 86:283–287.
Article24. Treves S, Anderson AA, Ducreux S, Divet A, Bleunven C, Grasso C, et al. Ryanodine receptor 1 mutations, dysregulation of calcium homeostasis and neuromuscular disorders. Neuromuscul Disord. 2005. 15:577–587.
Article25. Lynch PJ, Krivosic-Horber R, Reyford H, Monnier N, Quane K, Adnet P, et al. Identification of heterozygous and homozygous individuals with the novel RYR1 mutation Cys35Arg in a large kindred. Anesthesiology. 1997. 86:620–626.
Article26. Rueffert H, Olthoff D, Deutrich C, Thamm B, Froster UG. Homozygous and heterozygous Arg614Cys mutations (1840C-->T) in the ryanodine receptor gene co-segregate with malignant hyperthermia susceptibility in a German family. Br J Anaesth. 2001. 87:240–245.
Article27. Guis S, Figarella-Branger D, Monnier N, Bendahan D, Kozak-Ribbens G, Mattei JP, et al. Multiminicore disease in a family susceptible to malignant hyperthermia: histology, in vitro contracture tests, and genetic characterization. Arch Neurol. 2004. 61:106–113.
Article28. Shuaib A, Paasuke RT, Brownell KW. Central core disease. Clinical features in 13 patients. Medicine (Baltimore). 1987. 66:389–396.29. Sei Y, Sambuughin N, Muldoon S. Malignant hyperthermia genetic testing in North America Working Group Meeting. Bethesda, Maryland. September 4-5, 2002. Anesthesiology. 2004. 100:464–465.
Article30. Gillard EF, Otsu K, Fujii J, Duff C, de Leon S, Khanna VK, et al. Polymorphisms and deduced amino acid substitutions in the coding sequence of the ryanodine receptor (RYR1) gene in individuals with malignant hyperthermia. Genomics. 1992. 13:1247–1254.
Article31. McCarthy TV, Quane KA, Lynch PJ. Ryanodine receptor mutations in malignant hyperthermia and central core disease. Hum Mutat. 2000. 15:410–417.
Article32. Tilgen N, Zorzato F, Halliger-Keller B, Muntoni F, Sewry C, Palmucci LM, et al. Identification of four novel mutations in the C-terminal membrane spanning domain of the ryanodine receptor 1: association with central core disease and alteration of calcium homeostasis. Hum Mol Genet. 2001. 10:2879–2887.
Article33. Monnier N, Kozak-Ribbens G, Krivosic-Horber R, Nivoche Y, Qi D, Kraev N, et al. Correlations between genotype and pharmacological, histological, functional, and clinical phenotypes in malignant hyperthermia susceptibility. Hum Mutat. 2005. 26:413–425.
Article34. Shepherd S, Ellis F, Halsall J, Hopkins P, Robinson R. RYR1 mutations in UK central core disease patients: more than just the C-terminal transmembrane region of the RYR1 gene. J Med Genet. 2004. 41:e33.
Article35. Mathews KD, Moore SA. Multiminicore myopathy, central core disease, malignant hyperthermia susceptibility, and RYR1 mutations: one disease with many faces? Arch Neurol. 2004. 61:27–29.
Article36. Lamont PJ, Dubowitz V, Landon DN, Davis M, Morgan-Hughes JA. Fifty year follow-up of a patient with central core disease shows slow but definite progression. Neuromuscul Disord. 1998. 8:385–391.
Article37. Kraev N, Loke JC, Kraev A, MacLennan DH. Protocol for the sequence analysis of ryanodine receptor subtype 1 gene transcripts from human leukocytes. Anesthesiology. 2003. 99:289–296.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Two Cases of Evans Myopathy in a Family
- Delayed Onset of Malignant Hyperthermia: A Case Report
- Identification of G7304A Mutation in the Ryanodine Receptor Type 1 Gene in a Patient with Malignant Hyperthermia and an Extended Pedigree Study in a Korean Malignant Hyperthermia Family
- A Family of Congenital Fiber Type Disproportion with Mutation in Tropomyosin 3 (TPM3) Gene Presenting as Altered Mentality with Respiratory Distress
- Molecular Genetic Analysis of the Ryanodine Receptor Gene (RYR1) in Korean Malignant Hyperthermia Families