J Clin Neurol.
2018 Jan;14(1):29-34. 10.3988/jcn.2018.14.1.29.
Different Associations of Plasma Biomarkers in Alzheimer's Disease, Mild Cognitive Impairment, Vascular Dementia, and Ischemic Stroke
- Affiliations
-
- 1Department of Neurology, Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama University, Okayama, Japan. p4wi2ykv@okayama-u.ac.jp
- KMID: 2399596
- DOI: http://doi.org/10.3988/jcn.2018.14.1.29
Abstract
- BACKGROUND AND PURPOSE
Cognitive and cerebrovascular diseases are common in the elderly, but differences in the plasma levels and associations of plasma biomarkers in these diseases remain elusive.
METHODS
The present study investigated differences in plasma fatty acids [eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)], adiponectin, reptin, plasma markers of inflammation [high-sensitivity C-reactive protein (hsCRP) and serum amyloid A (serum AA)], and plasma lipids [high-density lipoprotein and low-density lipoprotein (LDL)] in patients with Alzheimer's disease (AD) (n=266), mild cognitive impairment (MCI) (n=44), vascular dementia (VaD) (n=33), and ischemic stroke (IS) (n=200) in comparison to normal controls (n=130).
RESULTS
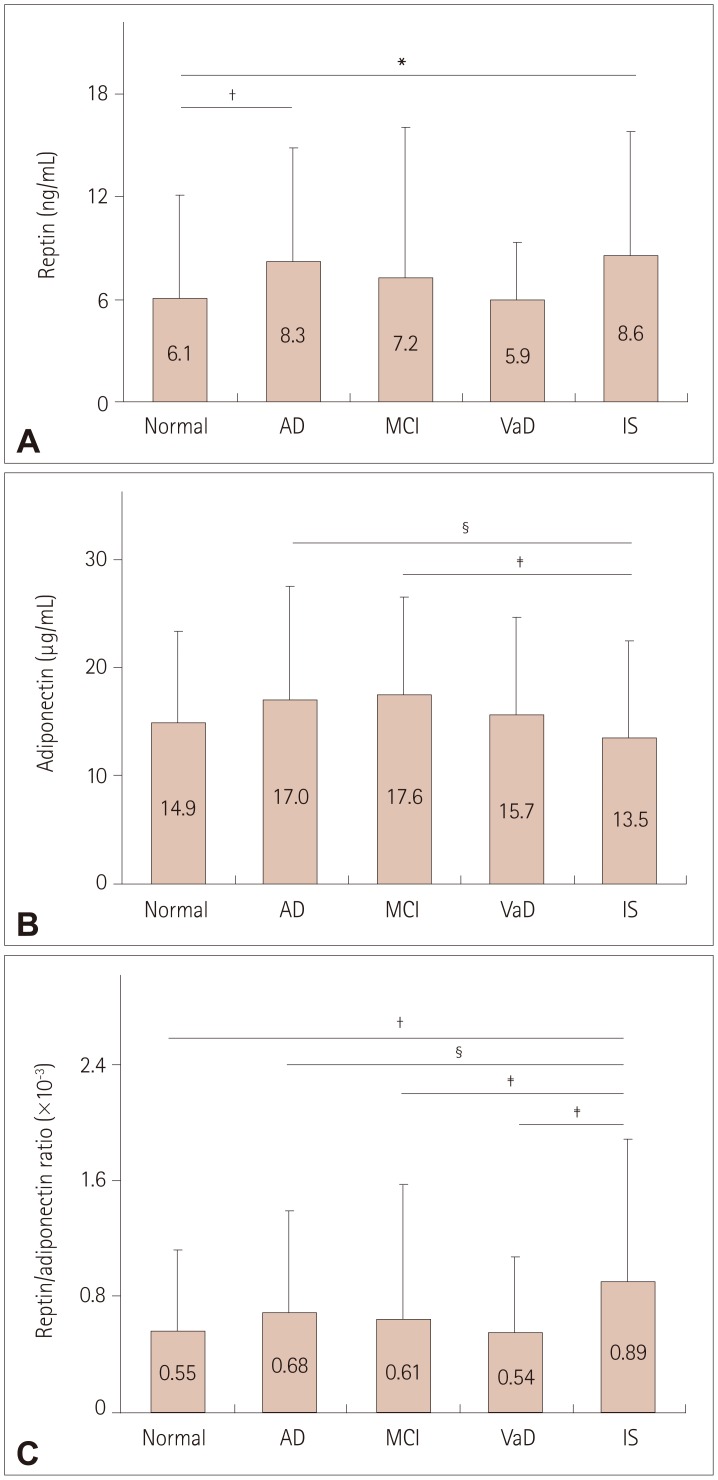
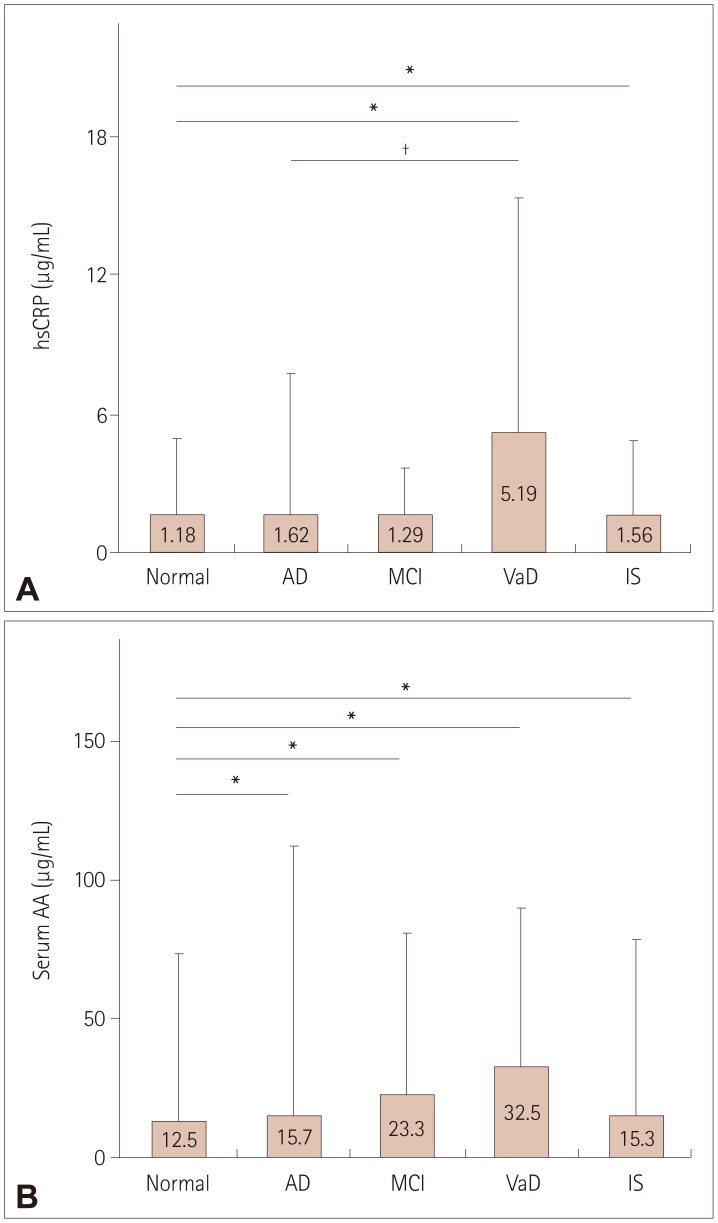
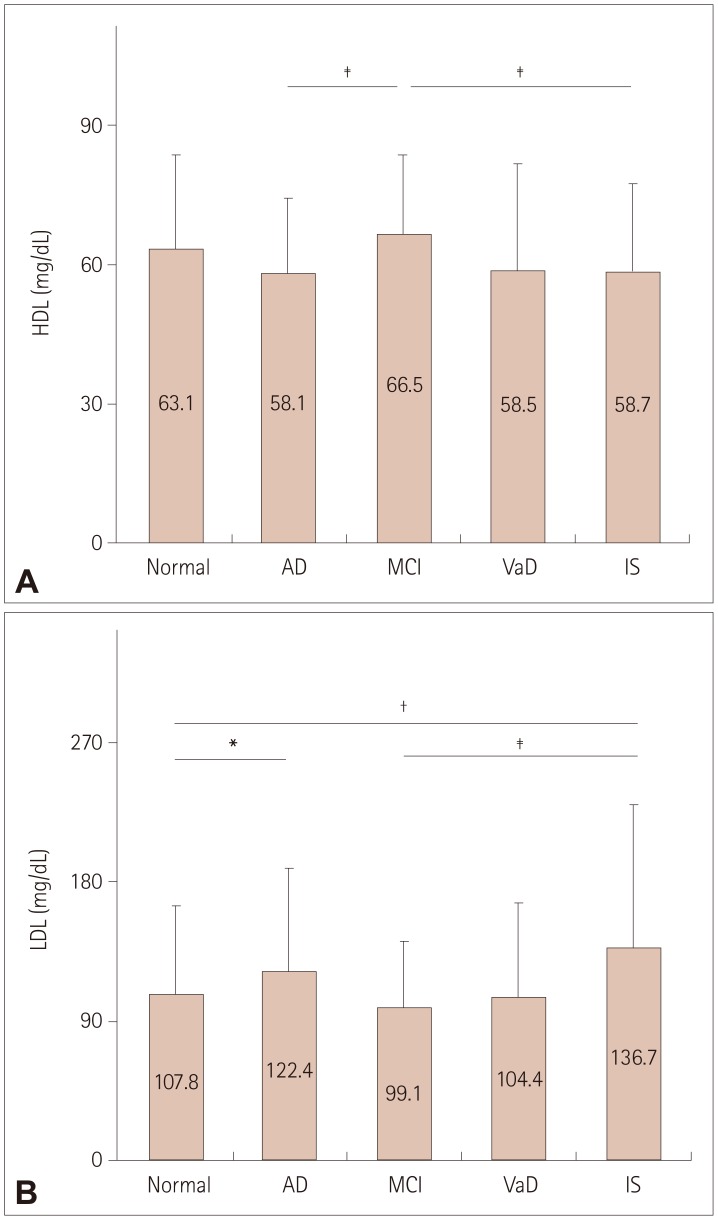
The serological data showed that lower EPA and DHA levels and higher reptin and LDL levels were associated with AD and IS, the reptin/adiponectin ratio was strongly associated with IS, the hsCRP level was more strongly associated with VaD and IS, and the serum AA level was associated with all three cognitive diseases and IS.
CONCLUSIONS
This is the first report of differences in the expression levels of plasma biomarkers and peripheral arterial tonometry among AD, MCI, VaD, and IS patients and normal controls. These different associations indicate that diverse pathological mechanisms underlie these diseases.
Keyword
MeSH Terms
-
Adiponectin
Aged
Alzheimer Disease*
Biomarkers*
C-Reactive Protein
Cerebrovascular Disorders
Dementia, Vascular*
Fatty Acids
Humans
Inflammation
Lipoproteins
Manometry
Mild Cognitive Impairment*
Plasma*
Serum Amyloid A Protein
Stroke*
Adiponectin
Biomarkers
C-Reactive Protein
Fatty Acids
Lipoproteins
Serum Amyloid A Protein
Figure
Reference
-
1. Kraal JJ, Peek N, van den Akker-Van Marle ME, Kemps HM. Effects and costs of home-based training with telemonitoring guidance in low to moderate risk patients entering cardiac rehabilitation: The FIT@Home study. BMC Cardiovasc Disord. 2013; 13:82. PMID: 24103384.
Article2. Hishikawa N, Fukui Y, Sato K, Kono S, Yamashita T, Ohta Y, et al. Characteristic features of cognitive, affective and daily living functions of late-elderly dementia. Geriatr Gerontol Int. 2016; 16:458–465. PMID: 25952646.
Article3. Rizzi L, Rosset I, Roriz-Cruz M. Global epidemiology of dementia: Alzheimer's and vascular types. Biomed Res Int. 2014; 2014:908915. PMID: 25089278.
Article4. Lopez OL. Mild cognitive impairment. Continuum (Minneap Minn). 2013; 19(2 Dementia):411–424. PMID: 23558486.
Article5. Takashima N, Arima H, Kita Y, Fujii T, Miyamatsu N, Komori M, et al. Incidence, management and short-term outcome of stroke in a general population of 1.4 million Japanese–Shiga Stroke Registry. Circ J. 2017; 81:1636–1646. PMID: 28579600.6. Kume A, Kurotani K, Sato M, Ejima Y, Pham NM, Nanri A, et al. Polyunsaturated fatty acids in serum and homocysteine concentrations in Japanese men and women: a cross-sectional study. Nutr Metab (Lond). 2013; 10:41. PMID: 23758810.
Article7. Yurko-Mauro K. Cognitive and cardiovascular benefits of docosahexaenoic acid in aging and cognitive decline. Curr Alzheimer Res. 2010; 7:190–196. PMID: 20088810.
Article8. Diop SB, Bertaux K, Vasanthi D, Sarkeshik A, Goirand B, Aragnol D, et al. Reptin and Pontin function antagonistically with PcG and TrxG complexes to mediate Hox gene control. EMBO Rep. 2008; 9:260–266. PMID: 18259215.
Article9. Stojanović S, Ilić MD, Ilić S, Petrović D, Djukić S. The significance of adiponectin as a biomarker in metabolic syndrome and/or coronary artery disease. Vojnosanit Pregl. 2015; 72:779–784. PMID: 26554109.
Article10. Kliper E, Bashat DB, Bornstein NM, Shenhar-Tsarfaty S, Hallevi H, Auriel E, et al. Cognitive decline after stroke: relation to inflammatory biomarkers and hippocampal volume. Stroke. 2013; 44:1433–1435. PMID: 23444307.11. Urieli-Shoval S, Linke RP, Matzner Y. Expression and function of serum amyloid A, a major acute-phase protein, in normal and disease states. Curr Opin Hematol. 2000; 7:64–69. PMID: 10608507.
Article12. Hottman DA, Chernick D, Cheng S, Wang Z, Li L. HDL and cognition in neurodegenerative disorders. Neurobiol Dis. 2014; 72 Pt A:22–36. PMID: 25131449.
Article13. Stampfer MJ. Cardiovascular disease and Alzheimer's disease: common links. J Intern Med. 2006; 260:211–223. PMID: 16918818.
Article14. McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011; 7:263–269. PMID: 21514250.
Article15. DeLaPaz RL, Wippold FJ 2nd, Cornelius RS, Amin-Hanjani S, Angtuaco EJ, Broderick DF, et al. ACR Appropriateness Criteria® on cerebrovascular disease. J Am Coll Radiol. 2011; 8:532–538. PMID: 21807345.
Article16. Simopoulos AP. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients. 2016; 8:128. PMID: 26950145.
Article17. Kim M, Nam JH, Oh DH, Park Y. Erythrocyte α-linolenic acid is associated with the risk for mild dementia in Korean elderly. Nutr Res. 2010; 30:756–761. PMID: 21130294.
Article18. Yurko-Mauro K, McCarthy D, Rom D, Nelson EB, Ryan AS, Blackwell A, et al. Beneficial effects of docosahexaenoic acid on cognition in age-related cognitive decline. Alzheimers Dement. 2010; 6:456–464. PMID: 20434961.19. Grigoletto A, Lestienne P, Rosenbaum J. The multifaceted proteins Reptin and Pontin as major players in cancer. Biochim Biophys Acta. 2011; 1815:147–157. PMID: 21111787.
Article20. Song J, Lee WT, Park KA, Lee JE. Association between risk factors for vascular dementia and adiponectin. Biomed Res Int. 2014; 2014:261672. PMID: 24860814.
Article21. Sener U, Uludag IF, Kose S, Ozcelik M, Zorlu Y. Is adiponectin a risk factor for transient ischaemic attacks? Endokrynol Pol. 2015; 66:214–218. PMID: 26136129.22. Khemka VK, Bagchi D, Bandyopadhyay K, Bir A, Chattopadhyay M, Biswas A, et al. Altered serum levels of adipokines and insulin in probable Alzheimer's disease. J Alzheimers Dis. 2014; 41:525–533. PMID: 24625795.
Article23. Shimabukuro M, Higa N, Asahi T, Oshiro Y, Takasu N, Tagawa T, et al. Hypoadiponectinemia is closely linked to endothelial dysfunction in man. J Clin Endocrinol Metab. 2003; 88:3236–3240. PMID: 12843170.
Article24. Sasaki M, Otani T, Kawakami M, Ishikawa SE. Elevation of plasma retinol-binding protein 4 and reduction of plasma adiponectin in subjects with cerebral infarction. Metabolism. 2010; 59:527–532. PMID: 19846170.
Article25. Chen MP, Tsai JC, Chung FM, Yang SS, Hsing LL, Shin SJ, et al. Hypoadiponectinemia is associated with ischemic cerebrovascular disease. Arterioscler Thromb Vasc Biol. 2005; 25:821–826. PMID: 15692106.
Article26. Hu SL, Xiong W, Dai ZQ, Zhao HL, Feng H. Cognitive changes during prolonged stay at high altitude and its correlation with C-reactive protein. PLoS One. 2016; 11:e0146290. PMID: 26731740.
Article27. Dullaart RP. Increased coronary heart disease risk determined by high high-density lipoprotein cholesterol and C-reactive protein: modulation by variation in the CETP gene. Arterioscler Thromb Vasc Biol. 2010; 30:1502–1503. PMID: 20631348.28. Yokoyama S. Unique features of high-density lipoproteins in the Japanese: in population and in genetic factors. Nutrients. 2015; 7:2359–2381. PMID: 25849946.
Article29. Zanchetti A, Liu L, Mancia G, Parati G, Grassi G, Stramba-Badiale M, et al. Blood pressure and low-density lipoprotein-cholesterol lowering for prevention of strokes and cognitive decline: a review of available trial evidence. J Hypertens. 2014; 32:1741–1750. PMID: 24979302.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Diagnosis and Differential Diagnosis of Dementia
- Recent Updates on Subcortical Ischemic Vascular Dementia
- Prevention and Treatment of Vascular Dementia
- The Clinical Significance of Cognitive Interventions for the Patients with Mild Cognitive Impairment
- Treatment of Vascular Dementia: A Comprehensive Review