Korean J Physiol Pharmacol.
2018 Jan;22(1):35-42. 10.4196/kjpp.2018.22.1.35.
Alleviation of ascorbic acid-induced gastric high acidity by calcium ascorbate in vitro and in vivo
- Affiliations
-
- 1Department of Physical Pharmacy, College of Pharmacy, Chungnam National University, Daejeon 34134, Korea. eicosa@cnu.ac.kr
- 2Department of Pharmacology, College of Pharmacy, Chungnam National University, Daejeon 34134, Korea. cm8r@cnu.ac.kr
- 3PHARMCROSS Co., Ltd., Chuncheon 24398, Korea.
- KMID: 2398553
- DOI: http://doi.org/10.4196/kjpp.2018.22.1.35
Abstract
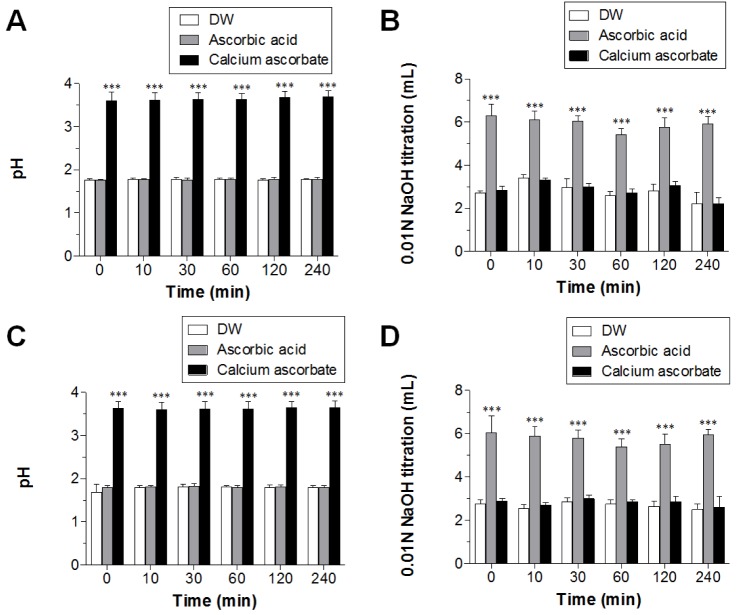
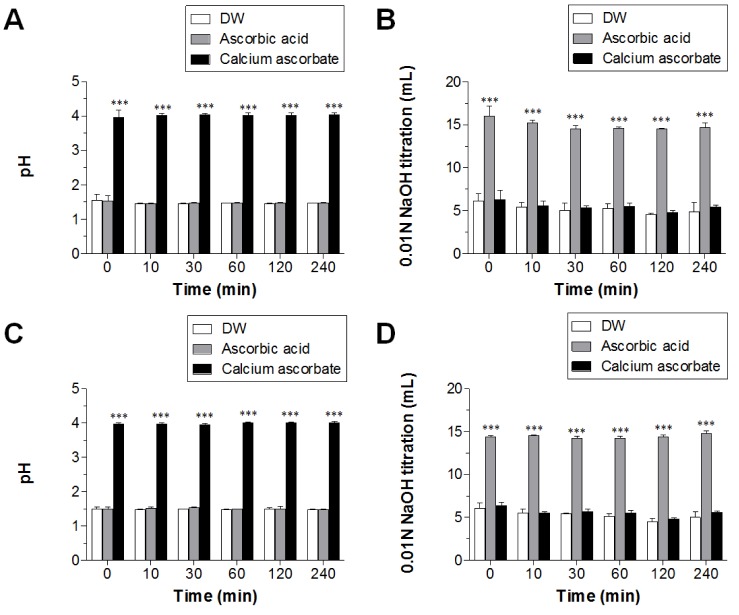
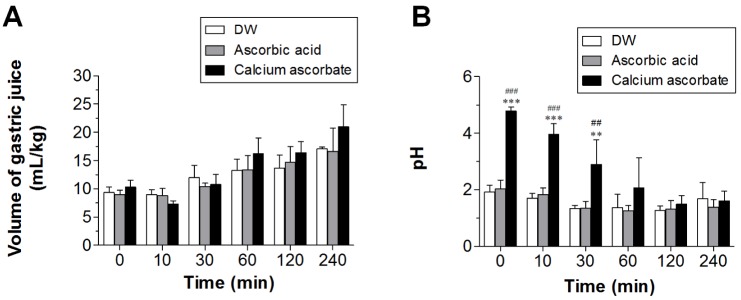
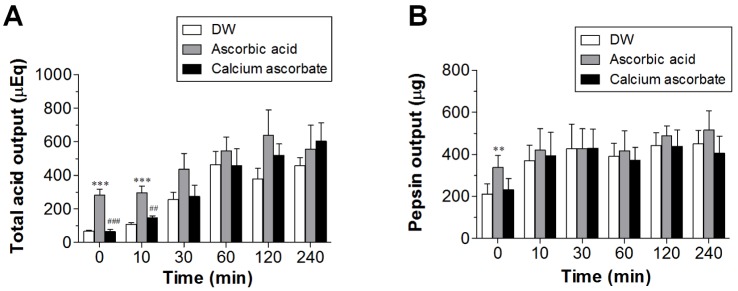
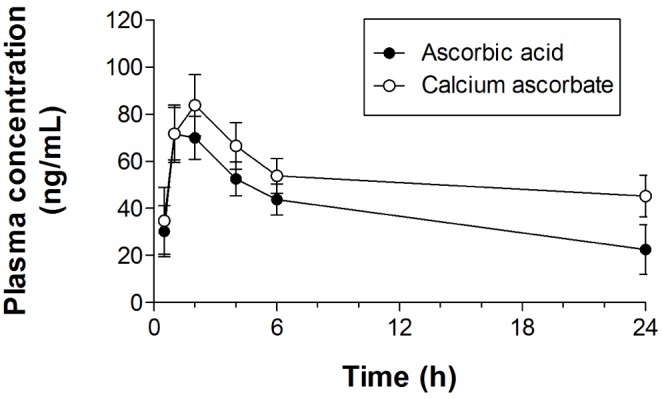
- Ascorbic acid is one of the most well-known nutritional supplement and antioxidant found in fruits and vegetables. Calcium ascorbate has been developed to mitigate the gastric irritation caused by the acidity of ascorbic acid. The aim of this study was to compare calcium ascorbate and ascorbic acid, focusing on their antioxidant activity and effects on gastric juice pH, total acid output, and pepsin secretion in an in vivo rat model, as well as pharmacokinetic parameters. Calcium ascorbate and ascorbic acid had similar antioxidant activity. However, the gastric fluid pH was increased by calcium ascorbate, whereas total acid output was increased by ascorbic acid. In the rat pylorus ligation-induced ulcer model, calcium ascorbate increased the gastric fluid pH without changing the total acid output. Administration of calcium ascorbate to rats given a single oral dose of 100 mg/kg as ascorbic acid resulted in higher plasma concentrations than that from ascorbic acid alone. The area under the curve (AUC) values of calcium ascorbate were 1.5-fold higher than those of ascorbic acid, and the C(max) value of calcium ascorbate (91.0 ng/ml) was higher than that of ascorbic acid (74.8 ng/ml). However, their T(max) values were similar. Thus, although calcium ascorbate showed equivalent antioxidant activity to ascorbic acid, it could attenuate the gastric high acidity caused by ascorbic acid, making it suitable for consideration of use to improve the side effects of ascorbic acid. Furthermore, calcium ascorbate could be an appropriate antioxidant substrate, with increased oral bioavailability, for patients with gastrointestinal disorders.
MeSH Terms
Figure
Reference
-
1. Valdes F. Vitamin C. Actas Dermosifiliogr. 2006; 97:557–568. PMID: 17173758.2. Naidu KA. Vitamin C in human health and disease is still a mystery? An overview. Nutr J. 2003; 2:7. PMID: 14498993.
Article3. Padayatty SJ, Katz A, Wang Y, Eck P, Kwon O, Lee JH, Chen S, Corpe C, Dutta A, Dutta SK, Levine M. Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr. 2003; 22:18–35. PMID: 12569111.
Article4. Carr A, Frei B. Does vitamin C act as a pro-oxidant under physiological conditions? FASEB J. 1999; 13:1007–1024. PMID: 10336883.
Article5. Martin A, Frei B. Both intracellular and extracellular vitamin C inhibit atherogenic modification of LDL by human vascular endothelial cells. Arterioscler Thromb Vasc Biol. 1997; 17:1583–1590. PMID: 9301639.
Article6. Padayatty SJ, Levine M. New insights into the physiology and pharmacology of vitamin C. CMAJ. 2001; 164:353–355. PMID: 11232136.7. Johnston CS. Biomarkers for establishing a tolerable upper intake level for vitamin C. Nutr Rev. 1999; 57:71–77. PMID: 10101920.
Article8. Anderson JW, Gowri MS, Turner J, Nichols L, Diwadkar VA, Chow CK, Oeltgen PR. Antioxidant supplementation effects on low-density lipoprotein oxidation for individuals with type 2 diabetes mellitus. J Am Coll Nutr. 1999; 18:451–461. PMID: 10511327.
Article9. Kim YY, Ku SY, Huh Y, Liu HC, Kim SH, Choi YM, Moon SY. Antiaging effects of vitamin C on human pluripotent stem cell-derived cardiomyocytes. Age (Dordr). 2013; 35:1545–1557. PMID: 22843416.
Article10. Hamrick I, Counts SH. Vitamin and mineral supplements. Prim Care. 2008; 35:729–747. PMID: 18928827.
Article11. Wilson JX. Regulation of vitamin C transport. Annu Rev Nutr. 2005; 25:105–125. PMID: 16011461.
Article12. Chen X, Shen L, Gu X, Dai X, Zhang L, Xu Y, Zhou P. High-dose supplementation with vitamin C-induced pediatric urolithiasis: the first case report in a child and literature review. Urology. 2014; 84:922–924. PMID: 25260453.
Article13. Sestili MA. Possible adverse health effects of vitamin C and ascorbic acid. Semin Oncol. 1983; 10:299–304. PMID: 6364356.14. Bush MJ, Verlangieri AJ. An acute study on the relative gastro-intestinal absorption of a novel form of calcium ascorbate. Res Commun Chem Pathol Pharmacol. 1987; 57:137–140. PMID: 3671876.15. Gruenwald J, Graubaum HJ, Busch R, Bentley C. Safety and tolerance of ester-C compared with regular ascorbic acid. Adv Ther. 2006; 23:171–178. PMID: 16644619.
Article16. Ozeki T, Mizuno S, Ohuchi H, Iwaki K, Watanabe S, Ueda H, Kawahara H, Masuda H, Sanefugi H. The effects of prostaglandin E1 on the pepsin activities in gastric mucosa and juice. Br J Exp Pathol. 1987; 68:521–526. PMID: 3115286.17. Guldvog I, Berstad A. Opposite effects of H2-receptors on parietal cells and chief cells. Gastric acid and pepsin secretion stimulated by histamine and food combined in dogs: the role of vagal innervation. Eur Surg Res. 1984; 16(Suppl 2):55–61. PMID: 6426965.18. Sipponen P, Maaroos HI. Chronic gastritis. Scand J Gastroenterol. 2015; 50:657–667. PMID: 25901896.
Article19. Katz PO. Lessons learned from intragastric pH monitoring. J Clin Gastroenterol. 2001; 33:107–113. PMID: 11468435.
Article20. Heimer KA, Hart AM, Martin LG, Rubio-Wallace S. Examining the evidence for the use of vitamin C in the prophylaxis and treatment of the common cold. J Am Acad Nurse Pract. 2009; 21:295–300. PMID: 19432914.
Article21. Vertzoni M, Dressman J, Butler J, Hempenstall J, Reppas C. Simulation of fasting gastric conditions and its importance for the in vivo dissolution of lipophilic compounds. Eur J Pharm Biopharm. 2005; 60:413–417. PMID: 15893920.
Article22. McConnell EL, Basit AW, Murdan S. Measurements of rat and mouse gastrointestinal pH, fluid and lymphoid tissue, and implications for in-vivo experiments. J Pharm Pharmacol. 2008; 60:63–70. PMID: 18088506.
Article23. Rao CV, Vijayakumar M. Effect of quercetin, flavonoids and alphatocopherol, an antioxidant vitamin, on experimental reflux oesophagitis in rats. Eur J Pharmacol. 2008; 589:233–238. PMID: 18547560.24. Sairam K, Rao ChV, Babu MD, Kumar KV, Agrawal VK, K Goel RK. Antiulcerogenic effect of methanolic extract of Emblica officinalis: an experimental study. J Ethnopharmacol. 2002; 82:1–9. PMID: 12169398.
Article25. Pancorbo D, Vazquez C, Fletcher MA. Vitamin C-lipid metabolites: uptake and retention and effect on plasma C-reactive protein and oxidized LDL levels in healthy volunteers. Med Sci Monit. 2008; 14:CR547–CR551. PMID: 18971870.26. Spinola V, Llorent-Martinez EJ, Castilho PC. Determination of vitamin C in foods: current state of method validation. J Chromatogr A. 2014; 1369:2–17. PMID: 25441066.27. Chatterjee IB, Majumder AK, Nandi BK, Subramanian N. Synthesis and some major functions of vitamin C in animals. Ann N Y Acad Sci. 1975; 258:24–47. PMID: 1106297.
Article28. Levine M, Rumsey SC, Daruwala R, Park JB, Wang Y. Criteria and recommendations for vitamin C intake. JAMA. 1999; 281:1415–1423. PMID: 10217058.
Article29. Masri OA, Chalhoub JM, Sharara AI. Role of vitamins in gastrointestinal diseases. World J Gastroenterol. 2015; 21:5191–5209. PMID: 25954093.
Article30. Hunt RH. Importance of pH control in the management of GERD. Arch Intern Med. 1999; 159:649–657. PMID: 10218743.
Article31. Goldberg HI, Dodds WJ, Gee S, Montgomery C, Zboralske FF. Role of acid and pepsin in acute experimental esophagitis. Gastroenterology. 1969; 56:223–230. PMID: 4884956.
Article32. Kwiecien S, Jasnos K, Magierowski M, Sliwowski Z, Pajdo R, Brzozowski B, Mach T, Wojcik D, Brzozowski T. Lipid peroxidation, reactive oxygen species and antioxidative factors in the pathogenesis of gastric mucosal lesions and mechanism of protection against oxidative stress - induced gastric injury. J Physiol Pharmacol. 2014; 65:613–622. PMID: 25371520.33. DeMeester TR, Wernly JA, Bryant GH, Little AG, Skinner DB. Clinical and in vitro analysis of determinants of gastroesophageal competence. A study of the principles of antireflux surgery. Am J Surg. 1979; 137:39–46. PMID: 31808.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Prospects of Vitamin C in Cancer Therapy
- The Effect of Ascorbic acid on Endotoxin-induced Fibrosis
- Ascorbate Oxidase Minimizes Interference by High-Concentration Ascorbic Acid in Total Cholesterol Assays
- Effects of glutamate on dehydroascorbate uptake and Its enhanced vulnerability to the peroxidation in cerebral cortical slices
- Enhancement of Plasmacytoma Cell Growth by Ascorbic Acid is Mediated Via Glucose 6-phosphate Dehydrogenase