J Breast Cancer.
2017 Dec;20(4):378-385. 10.4048/jbc.2017.20.4.378.
Improved Model for Predicting Axillary Response to Neoadjuvant Chemotherapy in Patients with Clinically Node-Positive Breast Cancer
- Affiliations
-
- 1Division of Breast Surgery, Department of Surgery, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 2Division of Breast Surgery, Department of Surgery, Bucheon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Bucheon, Korea. bjsong@catholic.ac.kr
- KMID: 2398208
- DOI: http://doi.org/10.4048/jbc.2017.20.4.378
Abstract
- PURPOSE
Pathological complete response (pCR) of axillary lymph node (LN) is frequently achieved in patients with clinically node-positive breast cancer after neoadjuvant chemotherapy (NAC). Treatment of the axilla after NAC is not well established and the value of sentinel LN biopsy following NAC remains unclear. This study investigated the predictive value of axillary response following NAC and evaluated the predictive value of a model based on axillary response.
METHODS
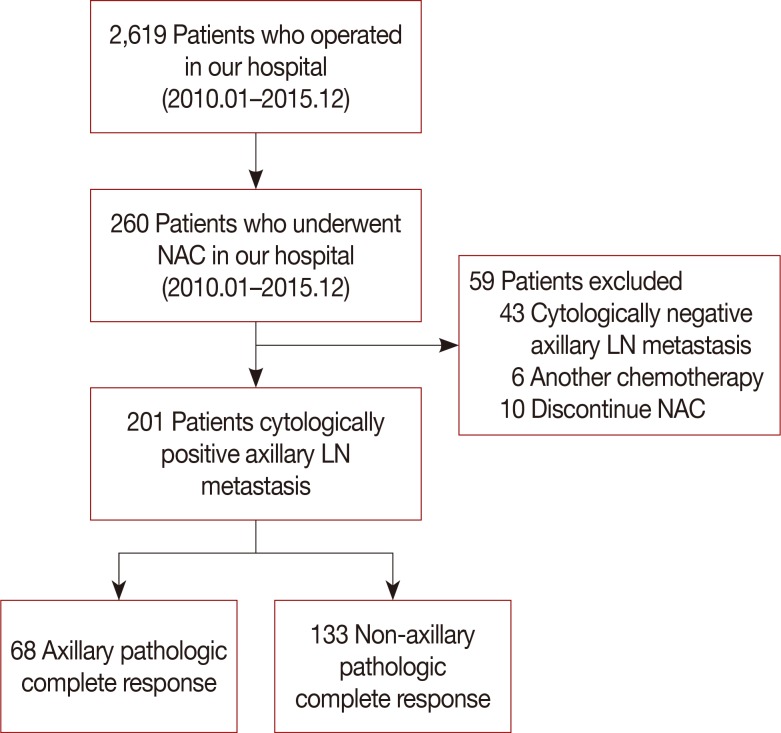
Data prospectively collected on 201 patients with clinically node-positive breast cancer who were treated with NAC and underwent axillary LN dissection (ALND) were retrieved. A model predictive of axillary pCR was developed based on clinicopathologic variables. The overall predictive ability between models was compared by receiver operating characteristic (ROC) curve analysis.
RESULTS
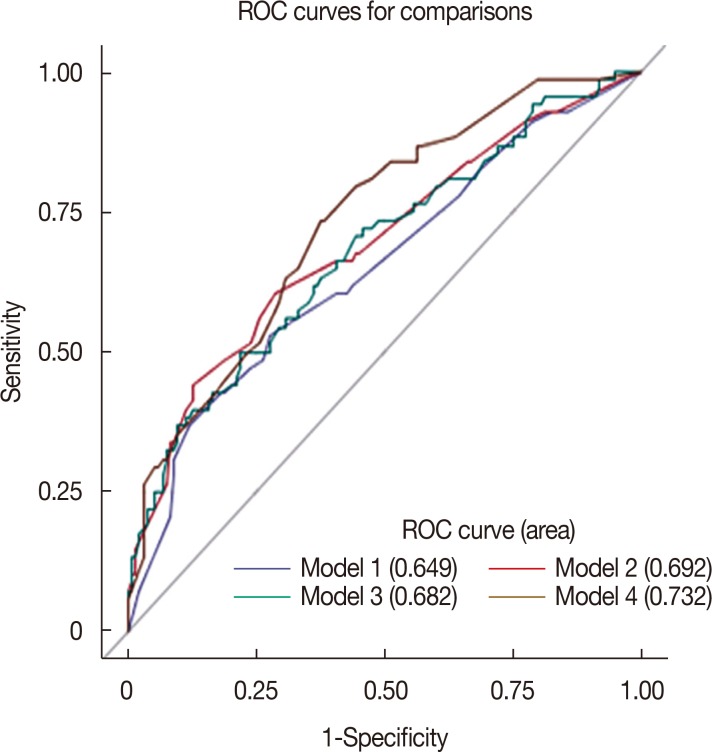
Of 201 patients who underwent ALND after NAC, 68 (33.8%) achieved axillary pCR. Multivariate analysis using axillary LN pCR after NAC as the dependent variable showed that higher histologic grade (p=0.031; odds ratio [OR], 2.537; 95% confidence interval [CI], 1.087-5.925) and tumor response rate ≥47.1% (p=0.001; OR, 3.212; 95% CI, 1.584-6.515) were significantly associated with an increased probability of achieving axillary pCR. The area under the ROC curve for estimating axillary pCR was significantly higher in the model that included tumor response rate than in the model that excluded this rate (0.732 vs. 0.649, p=0.022).
CONCLUSION
Tumor response rate was the most significant independent predictor of axillary pCR in response to NAC. The model that included tumor response rate was a significantly better predictor of axillary pCR than the model that excluded tumor response rate.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Targeted axillary biopsy and sentinel lymph node biopsy for axillary restaging after neoadjuvant chemotherapy
Gunay Gurleyik, Sibel Aydin Aksu, Fügen Aker, Kubra Kaytaz Tekyol, Eda Tanrikulu, Emin Gurleyik
Ann Surg Treat Res. 2021;100(6):305-312. doi: 10.4174/astr.2021.100.6.305.
Reference
-
1. Bear HD, Anderson S, Smith RE, Geyer CE Jr, Mamounas EP, Fisher B, et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2006; 24:2019–2027. PMID: 16606972.
Article2. Cunningham JD, Weiss SE, Ahmed S, Bratton JM, Bleiweiss IJ, Tartter PI, et al. The efficacy of neoadjuvant chemotherapy compared to postoperative therapy in the treatment of locally advanced breast cancer. Cancer Invest. 1998; 16:80–86. PMID: 9512673.
Article3. Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst. 2005; 97:188–194. PMID: 15687361.
Article4. Navarro-Cecilia J, Dueñas-Rodríguez B, Luque-López C, Ramírez-Expósito MJ, Martínez-Ferrol J, Ruíz-Mateas A, et al. Intraoperative sentinel node biopsy by one-step nucleic acid amplification (OSNA) avoids axillary lymphadenectomy in women with breast cancer treated with neoadjuvant chemotherapy. Eur J Surg Oncol. 2013; 39:873–879. PMID: 23711734.
Article5. Rouzier R, Mathieu MC, Sideris L, Youmsi E, Rajan R, Garbay JR, et al. Breast-conserving surgery after neoadjuvant anthracycline-based chemotherapy for large breast tumors. Cancer. 2004; 101:918–925. PMID: 15329898.
Article6. Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001; 30:96–102.
Article7. Gonzalez-Angulo AM, McGuire SE, Buchholz TA, Tucker SL, Kuerer HM, Rouzier R, et al. Factors predictive of distant metastases in patients with breast cancer who have a pathologic complete response after neoadjuvant chemotherapy. J Clin Oncol. 2005; 23:7098–7104. PMID: 16192593.
Article8. Hennessy BT, Hortobagyi GN, Rouzier R, Kuerer H, Sneige N, Buzdar AU, et al. Outcome after pathologic complete eradication of cytologically proven breast cancer axillary node metastases following primary chemotherapy. J Clin Oncol. 2005; 23:9304–9311. PMID: 16361629.
Article9. Rouzier R, Extra JM, Klijanienko J, Falcou MC, Asselain B, Vincent-Salomon A, et al. Incidence and prognostic significance of completeaxillary downstaging after primary chemotherapy in breast cancer patients with T1 to T3 tumors and cytologically proven axillary metastatic lymph nodes. J Clin Oncol. 2002; 20:1304–1310. PMID: 11870173.10. Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013; 310:1455–1461. PMID: 24101169.11. Lucci A, McCall LM, Beitsch PD, Whitworth PW, Reintgen DS, Blumencranz PW, et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol. 2007; 25:3657–3663. PMID: 17485711.
Article12. Mansel RE, Fallowfield L, Kissin M, Goyal A, Newcombe RG, Dixon JM, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst. 2006; 98:599–609. PMID: 16670385.
Article13. Kuehn T, Bauerfeind I, Fehm T, Fleige B, Hausschild M, Helms G, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013; 14:609–618. PMID: 23683750.
Article14. Jin X, Jiang YZ, Chen S, Shao ZM, Di GH. A nomogram for predicting the pathological response of axillary lymph node metastasis in breast cancer patients. Sci Rep. 2016; 6:32585. PMID: 27576704.
Article15. Vila J, Mittendorf EA, Farante G, Bassett RL, Veronesi P, Galimberti V, et al. Nomograms for predicting axillary response to neoadjuvant chemotherapy in clinically node-positive patients with breast cancer. Ann Surg Oncol. 2016; 23:3501–3509. PMID: 27216742.
Article16. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–247. PMID: 19097774.
Article17. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000; 92:205–216. PMID: 10655437.18. Alvarado R, Yi M, Le-Petross H, Gilcrease M, Mittendorf EA, Bedrosian I, et al. The role for sentinel lymph node dissection after neoadjuvant chemotherapy in patients who present with node-positive breast cancer. Ann Surg Oncol. 2012; 19:3177–3184. PMID: 22772869.
Article19. Straver ME, Rutgers EJ, Russell NS, Oldenburg HS, Rodenhuis S, Wesseling J, et al. Towards rational axillary treatment in relation to neoadjuvant therapy in breast cancer. Eur J Cancer. 2009; 45:2284–2292. PMID: 19464164.
Article20. Vugts G, Maaskant-Braat AJ, de Roos WK, Voogd AC, Nieuwenhuijzen GA. Management of the axilla after neoadjuvant chemotherapy for clinically node positive breast cancer: a nationwide survey study in the Netherlands. Eur J Surg Oncol. 2016; 42:956–964. PMID: 27107791.
Article21. Fleissig A, Fallowfield LJ, Langridge CI, Johnson L, Newcombe RG, Dixon JM, et al. Post-operative arm morbidity and quality of life: results of the ALMANAC randomised trial comparing sentinel node biopsy with standard axillary treatment in the management of patients with early breast cancer. Breast Cancer Res Treat. 2006; 95:279–293. PMID: 16163445.
Article22. Kakuda JT, Stuntz M, Trivedi V, Klein SR, Vargas HI. Objective assessment of axillary morbidity in breast cancer treatment. Am Surg. 1999; 65:995–998. PMID: 10515551.23. Caudle AS, Yang WT, Krishnamurthy S, Mittendorf EA, Black DM, Gilcrease MZ, et al. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol. 2016; 34:1072–1078. PMID: 26811528.
Article24. You S, Kang DK, Jung YS, An YS, Jeon GS, Kim TH. Evaluation of lymph node status after neoadjuvant chemotherapy in breast cancer patients: comparison of diagnostic performance of ultrasound, MRI and 18F-FDG PET/CT. Br J Radiol. 2015; 88:20150143. PMID: 26110204.25. Partridge SC, Gibbs JE, Lu Y, Esserman LJ, Tripathy D, Wolverton DS, et al. MRI measurements of breast tumor volume predict response to neoadjuvant chemotherapy and recurrence-free survival. AJR Am J Roentgenol. 2005; 184:1774–1781. PMID: 15908529.
Article26. Schipper RJ, Moossdorff M, Nelemans PJ, Nieuwenhuijzen GA, de Vries B, Strobbe LJ, et al. A model to predict pathologic complete response of axillary lymph nodes to neoadjuvant chemo (immuno) therapy in patients with clinically node-positive breast cancer. Clin Breast Cancer. 2014; 14:315–322. PMID: 24548732.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prognostic Factors in Breast Cancer Patients Following Neoadjuvant Chemotherapy
- Prospective Evaluation of the Feasibility of Sentinel Lymph Node Biopsy in Breast Cancer Patients with Negative Axillary Conversion after Neoadjuvant Chemotherapy
- Correlation between Tumor Response to Neoadjuvant Chemotherapy and Patient Outcome in Breast Cancer
- Evaluation of the Role of Axillary Lymph Node Fine-Needle Aspiration Cytology in Early Breast Cancer With or Without Neoadjuvant Chemotherapy
- Sentinel Lymph Node Biopsy in Breast Cancer: A Clinical Review and Update