J Korean Ophthalmol Soc.
2017 Dec;58(12):1333-1340. 10.3341/jkos.2017.58.12.1333.
Effects of Human Serum on Human Corneal Epithelial Cells in Vitro
- Affiliations
-
- 1Department of Ophthalmology, Gyeongsang National University School of Medicine, Jinju, Korea.
- 2Department of Ophthalmology, Gyeongsang National University Changwon Hospital, Changwon, Korea.
- 3Danggam Choi Eye Clinic, Busan, Korea.
- 4Department of Ophthalmology, Pusan National University School of Medicine, Yangsan, Korea. jongsool@pusan.ac.kr
- KMID: 2397847
- DOI: http://doi.org/10.3341/jkos.2017.58.12.1333
Abstract
- PURPOSE
To investigate the effect of human serum on corneal epithelial cells.
METHODS
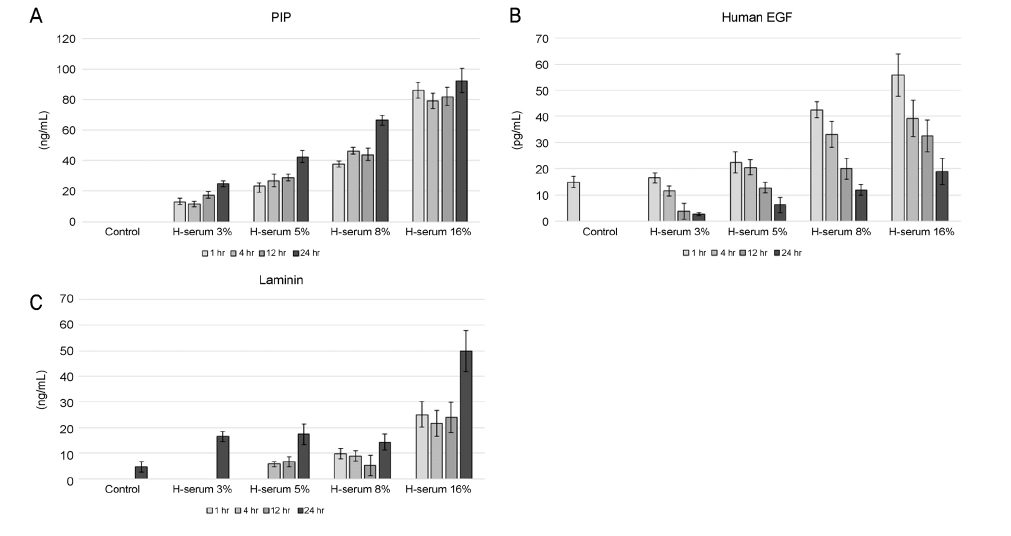
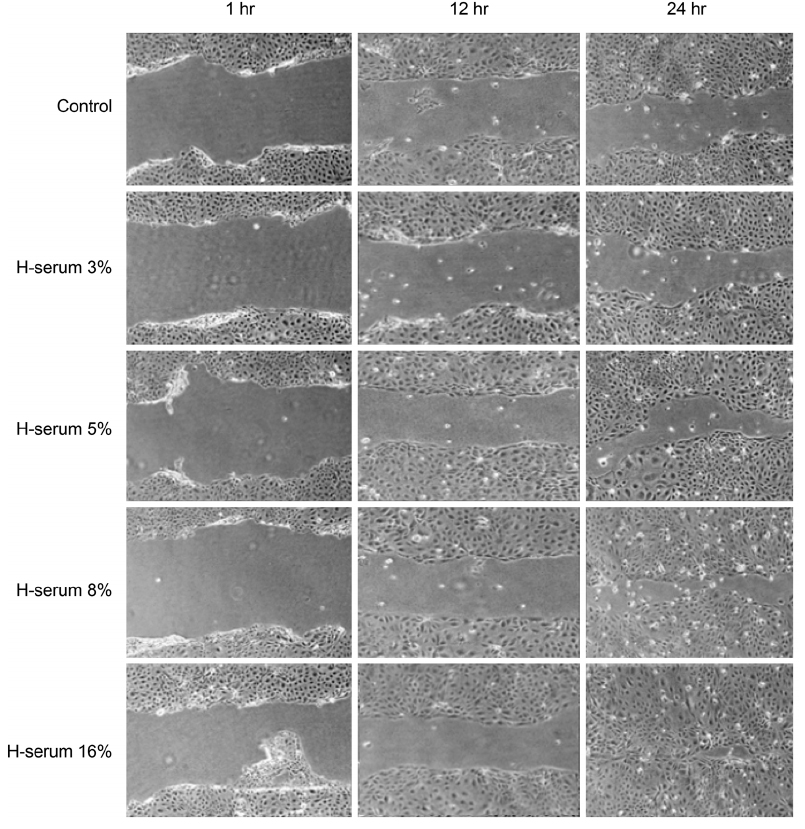
Changes of corneal epithelial cells were evaluated after 1, 4, 12, and 24 hours (hrs) of exposure to various concentrations of human serum (3, 5, 8, and 16%). Cellular metabolic activity and the extent of cellular damage were measured. Effect of human serum on cell migration was also examined. Concentration of procollagen type-I COOH-terminal peptide (PIP), epidermal growth factor (EGF), and laminin after exposure to human serum was further observed.
RESULTS
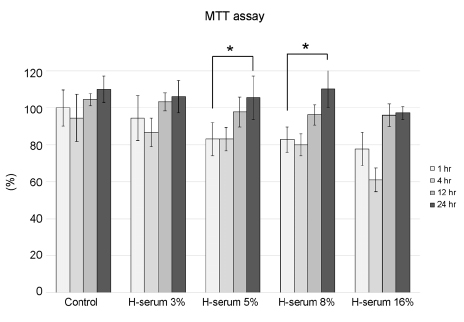
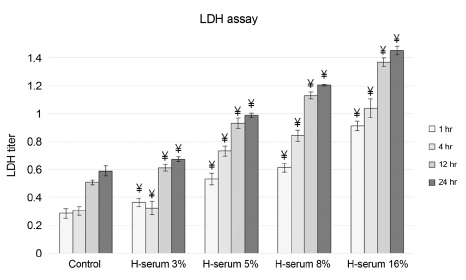
In every concentration of human serum, metabolic activity of the corneal epithelial cells temporarily decreased at 4 hrs of exposure and recovered to baseline levels afterward. With the same exposure time, there was no statistically significant difference in metabolic activity between the human serum-exposed group and the control group. Cellular toxicity of human serum exhibited a time- and dose-dependent relationship. Cellular migration was observed after 24 hrs of exposure to 5% concentration of human serum and after 12 hrs of exposure to 8% and 16% concentration of human serum. The PIP, EGF, and laminin titers increased in time- and dose-dependent manners.
CONCLUSIONS
Human serum does not decrease the metabolic activity of corneal epithelial cells as the concentration and exposure time increase, but it can induce cytotoxicity. Considering cellular migration, a serum concentration of 5% or higher should be used.
MeSH Terms
Figure
Reference
-
1. Bray RC, Leonard CA, Salo PT. Correlation of healing capacity with vascular response in the anterior cruciate and medial collateral ligaments of the rabbit. J Orthop Res. 2003; 21:1118–1123.2. Folkman J, Shing Y. Angiogenesis. J Biol Chem. 1992; 267:10931–10934.3. Hwang J, Chung SH, Jeon S, et al. Comparison of clinical efficacies of autologous serum eye drops in patients with primary and secondary Sjögren syndrome. Cornea. 2014; 33:663–667.4. Noble BA, Loh RS, MacLennan S, et al. Comparison of autologous serum eye drops with conventional therapy in a randomised controlled crossover trial for ocular surface disease. Br J Ophthalmol. 2004; 88:647–652.5. Fox RI, Chan R, Michelson JB, et al. Beneficial effect of artificial tears made with autologous serum in patients with keratoconjunctivitis sicca. Arthritis Rheum. 1984; 27:459–461.6. Kojima T, Higuchi A, Goto E, et al. Autologous serum eye drops for the treatment of dry eye diseases. Cornea. 2008; 27:Suppl 1. S25–S30.7. Matsumoto Y, Dogru M, Goto E, et al. Autologous serum application in the treatment of neurotrophic keratopathy. Ophthalmology. 2004; 111:1115–1120.8. Ogawa Y, Okamoto S, Mori T, et al. Autologous serum eye drops for the treatment of severe dry eye in patients with chronic graft-versus-host disease. Bone Marrow Transplant. 2003; 31:579–583.9. Phan TM, Foster CS, Boruchoff SA, et al. Topical fibronectin in the treatment of persistent corneal epithelial defects and trophic ulcers. Am J Ophthalmol. 1987; 104:494–501.10. Gipson IK, Grill SM. A technique for obtaining sheets of intact rabbit corneal epithelium. Invest Ophthalmol Vis Sci. 1982; 23:269–273.11. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65:55–63.12. Lee JS, Kim YH, Park YM. The toxicity of nonsteroidal anti-inflammatory eye drops against human corneal epithelial cells in vitro. J Korean Med Sci. 2015; 30:1856–1864.13. Park YM, Kim SJ, Han YS, Lee JS. Cellular toxicity of calf blood extract on human corneal epithelial cells in vitro. Curr Eye Res. 2015; 40:66–71.14. Timpl R, Rohde H, Robey PG, et al. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979; 254:9933–9937.15. Kwansa AL, De Vita R, Freeman JW. Mechanical recruitment of N- and C-crosslinks in collagen type I. Matrix Biol. 2014; 34:161–169.16. Couch JM, Cullen P, Casey TA, Fabre JW. Mitotic activity of corneal endothelial cells in organ culture with recombinant human epidermal growth factor. Ophthalmology. 1987; 94:1–6.17. Woost PG, Jumblatt MM, Eiferman RA, Schultz GS. Growth factors and corneal endothelial cells: I. Stimulation of bovine corneal endothelial cell DNA synthesis by defined growth factors. Cornea. 1992; 11:1–10.18. Brightwell JR, Riddle SL, Eiferman RA, et al. Biosynthetic human EGF accelerates healing of Neodecadron-treated primate corneas. Invest Ophthalmol Vis Sci. 1985; 26:105–110.19. Petroutsos G, Courty J, Guimaraes R, et al. Comparison of the effects of EGF, pFGF and EDGF on corneal epithelium wound healing. Curr Eye Res. 1984; 3:593–598.20. Ho PC, Davis WH, Elliott JH, Cohen S. Kinetics of corneal epithelial regeneration and epidermal growth factor. Invest Ophthalmol. 1974; 13:804–809.21. Kruse FE, Tseng SC. A serum-free clonal growth assay for limbal, peripheral, and central corneal epithelium. Invest Ophthalmol Vis Sci. 1991; 32:2086–2095.22. Watanabe K, Nakagawa S, Nishida T. Stimulatory effects of fibronectin and EGF on migration of corneal epithelial cells. Invest Ophthalmol Vis Sci. 1987; 28:205–211.23. Tsubota K, Goto E, Shimmura S, Shimazaki J. Treatment of persistent corneal epithelial defect by autologous serum application. Ophthalmology. 1999; 106:1984–1989.24. Jo HY, Kim Y, Park HW, et al. The unreliability of MTT assay in the cytotoxic test of primary cultured glioblastoma cells. Exp Neurobiol. 2015; 24:235–245.25. Lee CH, Park M, Kang SJ. The Effects of Calcium Nutrition on the Activities of Lactate Dehydrogenase, Alcohol Dehydrogenase and Other Enzymes in Melon (Cucumis melo L.) Seedlings Subjected to Flooding. Korean J Soil Sci Fert. 2016; 49:36–43.26. Saika S, Ohnishi Y, Ooshima A, et al. Epithelial repair: roles of extracellular matrix. Cornea. 2002; 21:2 Suppl 1. S23–S29.27. Handa JT, Murad S, Jaffe GJ. Minoxidil inhibits ocular cell proliferation and lysyl hydroxylase activity. Invest Ophthalmol Vis Sci. 1993; 34:567–575.28. Saika S, Yamanaka O, Hashizume N, et al. Cis-hydroxyproline inhibits adhesion, migration and proliferation of cultured rabbit keratocytes. Ophthalmic Res. 1993; 25:363–370.29. Tzu J, Marinkovich MP. Bridging structure with function: structural, regulatory, and developmental role of laminins. Int J Biochem Cell Biol. 2008; 40:199–214.30. Ebihara N, Mizushima H, Miyazaki K, et al. The functions of exogenous and endogenous laminin-5 on corneal epithelial cells. Exp Eye Res. 2000; 71:69–79.31. Hashimoto J, Kariya Y, Miyazaki K. Regulation of proliferation and chondrogenic differentiation of human mesenchymal stem cells by laminin-5 (laminin-332). Stem cells. 2006; 24:2346–2354.32. Kim HS, Jun Song X, de Paiva CS, et al. Phenotypic characterization of human corneal epithelial cells expanded ex vivo from limbal explant and single cell cultures. Exp Eye Res. 2004; 79:41–49.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Calf Serum on Human Corneal Epithelial Cells in Vitro
- Effects of Damaged Human Corneal Epithelial Cells on Differentiation of Human Mesenchymal Stem Cell
- Expression of HSV-1 Antigen in HSV-1 Infected Rabbit Corneal Cells in Vitro
- Bacterial Adherence to Human Buccal Epitheliald Cells and Its Possible Role in Bacterial Colonization in Human Oral Cavity
- Expression of Local Immunosuppressive Factor, Indoleamine 2,3-dixygenase, in Human Coreal Cells