Korean Circ J.
2017 Nov;47(6):795-810. 10.4070/kcj.2017.0300.
Bioresorbable Vascular Scaffold Korean Expert Panel Report
- Affiliations
-
- 1Heart Institute, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea. sjpark@amc.seoul.kr
- 2Division of Cardiology, Severance Cardiovascular Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 3Division of Cardiology, Department of Medicine, Chonnam National University Hospital, Gwangju, Korea.
- 4Division of Cardiology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 5Department of Cardiology, CHA Bundang Medical Center, CHA University, Seongnam, Korea.
- 6Department of Cardiology, Cardiovascular Center, Korea University Anam Hospital, Seoul, Korea.
- 7Department of Medicine, Keimyung University College of Medicine, Daegu, Korea.
- 8Department of Internal Medicine, Wonkwang University Sanbon Hospital, Sanbon, Korea.
- 9Division of Cardiology, Department of Internal Medicine, Sungkyunkwan University Samsung Changwon Hospital, Changwon, Korea.
- 10Division of Cardiology, Department of Internal Medicine, Kosin University Gospel Hospital, Busan, Korea.
- 11Department of Cardiology, Myongji Hospital, Goyang, Korea.
- 12Cardiovascular Center, Korea University Guro Hospital, Seoul, Korea.
- 13Division of Cardiology, The Catholic University of Korea, Daejeon St. Mary's Hospital, Daejeon, Korea.
- 14Department of Cardiology, Chung-Ang University Hospital, Seoul, Korea.
- 15Cardiovascular Center, GangNeung Asan Hospital, Gangneung, Korea.
- 16Division of Cardiology, Ajou University Medical Center, Suwon, Korea.
- 17Division of Cardiology, Department of Internal Medicine, Daegu Catholic University Medical Center, Daegu, Korea.
- 18Department of Cardiology, Dong-A University Medical Center, Busan, Korea.
- KMID: 2396472
- DOI: http://doi.org/10.4070/kcj.2017.0300
Abstract
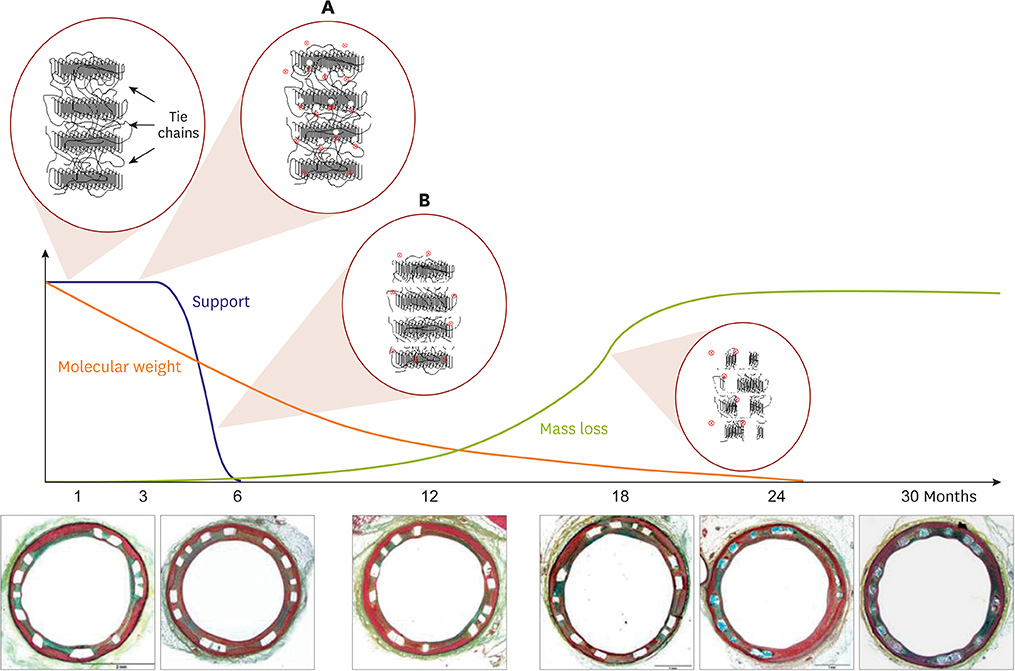
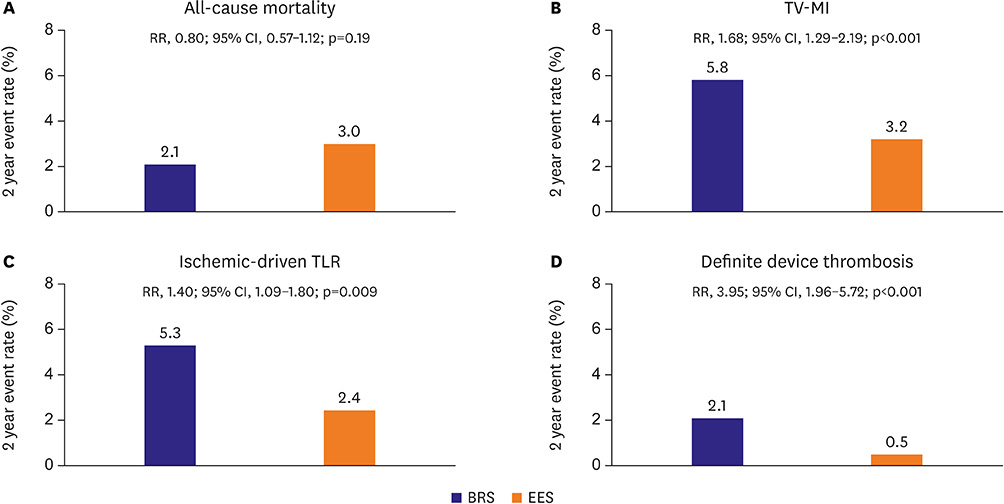
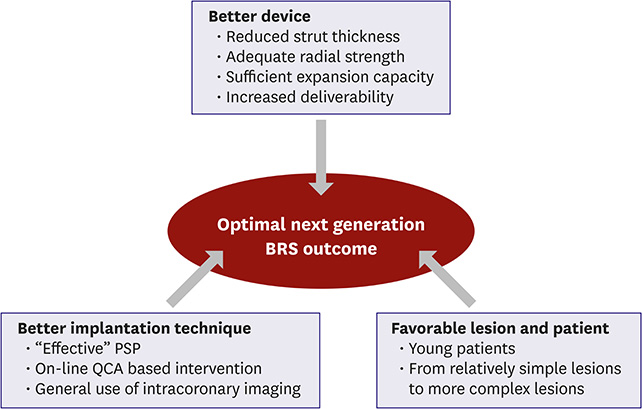
- Bioresorbable vascular scaffold (BRS) is an innovative device that provides structural support and drug release to prevent early recoil or restenosis, and then degrades into nontoxic compounds to avoid late complications related with metallic drug-eluting stents (DESs). BRS has several putative advantages. However, recent randomized trials and registry studies raised clinical concerns about the safety and efficacy of first generation BRS. In addition, the general guidance for the optimal practice with BRS has not been suggested due to limited long-term clinical data in Korea. To address the safety and efficacy of BRS, we reviewed the clinical evidence of BRS implantation, and suggested the appropriate criteria for patient and lesion selection, scaffold implantation technique, and management.
Figure
Reference
-
1. Serruys PW, Chevalier B, Dudek D, et al. A bioresorbable everolimus-eluting scaffold versus a metallic everolimus-eluting stent for ischaemic heart disease caused by de-novo native coronary artery lesions (ABSORB II): an interim 1-year analysis of clinical and procedural secondary outcomes from a randomised controlled trial. Lancet. 2015; 385:43–54.2. Karanasos A, Simsek C, Gnanadesigan M, et al. OCT assessment of the long-term vascular healing response 5 years after everolimus-eluting bioresorbable vascular scaffold. J Am Coll Cardiol. 2014; 64:2343–2356.3. Brugaletta S, Radu MD, Garcia-Garcia HM, et al. Circumferential evaluation of the neointima by optical coherence tomography after ABSORB bioresorbable vascular scaffold implantation: can the scaffold cap the plaque? Atherosclerosis. 2012; 221:106–112.4. Simsek C, Karanasos A, Magro M, et al. Long-term invasive follow-up of the everolimus-eluting bioresorbable vascular scaffold: five-year results of multiple invasive imaging modalities. EuroIntervention. 2016; 11:996–1003.5. Serruys PW, Chevalier B, Sotomi Y, et al. Comparison of an everolimus-eluting bioresorbable scaffold with an everolimus-eluting metallic stent for the treatment of coronary artery stenosis (ABSORB II): a 3 year, randomised, controlled, single-blind, multicentre clinical trial. Lancet. 2016; 388:2479–2491.6. Wykrzykowska JJ, Kraak RP, Hofma SH, et al. Bioresorbable scaffolds versus metallic stents in routine PCI. N Engl J Med. 2017; 376:2319–2328.7. Ellis SG, Kereiakes DJ, Metzger DC, et al. Everolimus-eluting bioresorbable scaffolds for coronary artery disease. N Engl J Med. 2015; 373:1905–1915.8. Tamburino C, Latib A, van Geuns RJ, et al. Contemporary practice and technical aspects in coronary intervention with bioresorbable scaffolds: a European perspective. EuroIntervention. 2015; 11:45–52.9. Foin N, Lee R, Bourantas C, et al. Bioresorbable vascular scaffold radial expansion and conformation compared to a metallic platform: insights from in vitro expansion in a coronary artery lesion model. EuroIntervention. 2016; 12:834–844.10. Onuma Y, Dudek D, Thuesen L, et al. Five-year clinical and functional multislice computed tomography angiographic results after coronary implantation of the fully resorbable polymeric everolimus-eluting scaffold in patients with de novo coronary artery disease: the ABSORB cohort A trial. JACC Cardiovasc Interv. 2013; 6:999–1009.11. Serruys PW, Ormiston J, van Geuns RJ, et al. A polylactide bioresorbable scaffold eluting everolimus for treatment of coronary stenosis: 5-year follow-up. J Am Coll Cardiol. 2016; 67:766–776.12. Ali ZA, Serruys PW, Kimura T, et al. 2-year outcomes with the absorb bioresorbable scaffold for treatment of coronary artery disease: a systematic review and meta-analysis of seven randomised trials with an individual patient data substudy. Lancet. 2017; 390:760–772.13. Capodanno D, Gori T, Nef H, et al. Percutaneous coronary intervention with everolimus-eluting bioresorbable vascular scaffolds in routine clinical practice: early and midterm outcomes from the European multicentre GHOST-EU registry. EuroIntervention. 2015; 10:1144–1153.14. Abizaid A, Ribamar Costa J Jr, Bartorelli AL, et al. The ABSORB EXTEND study: preliminary report of the twelve-month clinical outcomes in the first 512 patients enrolled. EuroIntervention. 2015; 10:1396–1401.15. Wiebe J, Hoppmann P, Colleran R, et al. Long-term clinical outcomes of patients treated with everolimus-eluting bioresorbable stents in routine practice: 2-year results of the ISAR-ABSORB Registry. JACC Cardiovasc Interv. 2017; 10:1222–1229.16. Cortese B, Ielasi A, Moscarella E, et al. Thirty-day outcomes after unrestricted implantation of bioresorbable vascular scaffold (from the prospective RAI Registry). Am J Cardiol. 2017; 119:1924–1930.17. Wöhrle J, Naber C, Schmitz T, et al. Beyond the early stages: insights from the ASSURE registry on bioresorbable vascular scaffolds. EuroIntervention. 2015; 11:149–156.18. Puricel S, Cuculi F, Weissner M, et al. Bioresorbable coronary scaffold thrombosis: multicenter comprehensive analysis of clinical presentation, mechanisms, and predictors. J Am Coll Cardiol. 2016; 67:921–931.19. Kraak RP, Hassell ME, Grundeken MJ, et al. Initial experience and clinical evaluation of the absorb bioresorbable vascular scaffold (BVS) in real-world practice: the AMC Single Centre Real World PCI Registry. EuroIntervention. 2015; 10:1160–1168.20. Rzeszutko Ł, Siudak Z, Tokarek T. Twelve months clinical outcome after bioresorbable vascular scaffold implantation in patients with stable angina and acute coronary syndrome. Data from the Polish National Registry. Postepy Kardiol Interwencyjnej. 2016; 12:108–115.21. Felix CM, Fam JM, Diletti R, et al. Mid- to long-term clinical outcomes of patients treated with the everolimus-eluting bioresorbable vascular scaffold: the BVS Expand Registry. JACC Cardiovasc Interv. 2016; 9:1652–1663.22. Muramatsu T, Onuma Y, van Geuns RJ, et al. 1-year clinical outcomes of diabetic patients treated with everolimus-eluting bioresorbable vascular scaffolds: a pooled analysis of the ABSORB and the SPIRIT trials. JACC Cardiovasc Interv. 2014; 7:482–493.23. Dudek D, Rzeszutko Ł, Zasada W, et al. Bioresorbable vascular scaffolds in patients with acute coronary syndromes: the POLAR ACS study. Pol Arch Med Wewn. 2014; 124:669–677.24. Gori T, Schulz E, Hink U, et al. Early outcome after implantation of absorb bioresorbable drug-eluting scaffolds in patients with acute coronary syndromes. EuroIntervention. 2014; 9:1036–1041.25. Hellenkamp K, Becker A, Gabriel YD, et al. Mid- to long-term outcome of patients treated with everolimus-eluting bioresorbable vascular scaffolds: data of the BVS registry Göttingen predominantly from ACS patients. Int J Cardiol. 2017; 234:58–63.26. Kajiya T, Liang M, Sharma RK, et al. Everolimus-eluting bioresorbable vascular scaffold (BVS) implantation in patients with ST-segment elevation myocardial infarction (STEMI). EuroIntervention. 2013; 9:501–504.27. Kočka V, Malý M, Toušek P, et al. Bioresorbable vascular scaffolds in acute ST-segment elevation myocardial infarction: a prospective multicentre study ‘Prague 19’. Eur Heart J. 2014; 35:787–794.28. Wiebe J, Möllmann H, Most A, et al. Short-term outcome of patients with ST-segment elevation myocardial infarction (STEMI) treated with an everolimus-eluting bioresorbable vascular scaffold. Clin Res Cardiol. 2014; 103:141–148.29. Diletti R, Karanasos A, Muramatsu T, et al. Everolimus-eluting bioresorbable vascular scaffolds for treatment of patients presenting with ST-segment elevation myocardial infarction: BVS STEMI first study. Eur Heart J. 2014; 35:777–786.30. Brugaletta S, Gori T, Low AF, et al. Absorb bioresorbable vascular scaffold versus everolimus-eluting metallic stent in ST-segment elevation myocardial infarction: 1-year results of a propensity score matching comparison: the BVS-EXAMINATION Study (bioresorbable vascular scaffold-a clinical evaluation of everolimus eluting coronary stents in the treatment of patients with ST-segment elevation myocardial infarction). JACC Cardiovasc Interv. 2015; 8:189–197.31. Cortese B, Ielasi A, Romagnoli E, et al. Clinical comparison with short-term follow-up of bioresorbable vascular scaffold versus everolimus-eluting stent in primary percutaneous coronary interventions. Am J Cardiol. 2015; 116:705–710.32. Kochman J, Tomaniak M, Pietrasik A, et al. Bioresorbable everolimus-eluting vascular scaffold in patients with ST-segment elevation myocardial infarction: optical coherence tomography evaluation and clinical outcomes. Cardiol J. 2015; 22:315–322.33. Chakraborty R, Patra S, Banerjee S, et al. Outcome of everolimus eluting bioabsorbable vascular scaffold (BVS) compared to non BVS drug eluting stent in the management of ST-segment elevation myocardial infarction (STEMI) - a comparative study. Cardiovasc Revasc Med. 2016; 17:151–154.34. Diletti R, Farooq V, Girasis C, et al. Clinical and intravascular imaging outcomes at 1 and 2 years after implantation of absorb everolimus eluting bioresorbable vascular scaffolds in small vessels. Late lumen enlargement: does bioresorption matter with small vessel size? Insight from the ABSORB cohort B trial. Heart. 2013; 99:98–105.35. Ielasi A, Latib A, Naganuma T, et al. Early results following everolimus-eluting bioresorbable vascular scaffold implantation for the treatment of in-stent restenosis. Int J Cardiol. 2014; 173:513–514.36. Moscarella E, Ielasi A, Granata F, et al. Long-term clinical outcomes after bioresorbable vascular scaffold implantation for the treatment of coronary in-stent restenosis: a multicenter Italian experience. Circ Cardiovasc Interv. 2016; 9:e003148.37. Alfonso F, Cuesta J, Pérez-Vizcayno MJ, et al. Bioresorbable vascular scaffolds for patients with in-stent restenosis: the RIBS VI Study. JACC Cardiovasc Interv. 2017; 10:1841–1851.38. Everaert B, Wykrzykowska JJ, Koolen J, et al. Recommendations for the use of bioresorbable vascular scaffolds in percutaneous coronary interventions: 2017 revision. Neth Heart J. 2017; 25:419–428.39. Indolfi C, De Rosa S, Colombo A. Bioresorbable vascular scaffolds - basic concepts and clinical outcome. Nat Rev Cardiol. 2016; 13:719–729.40. Miyazaki T, Latib A, Ruparelia N, et al. The use of a scoring balloon for optimal lesion preparation prior to bioresorbable scaffold implantation: a comparison with conventional balloon predilatation. EuroIntervention. 2016; 11:e1580–e1588.41. Hibi K, Kimura K, Umemura S. Clinical utility and significance of intravascular ultrasound and optical coherence tomography in guiding percutaneous coronary interventions. Circ J. 2015; 79:24–33.42. Kimura T, Kozuma K, Tanabe K, et al. A randomized trial evaluating everolimus-eluting absorb bioresorbable scaffolds vs. everolimus-eluting metallic stents in patients with coronary artery disease: ABSORB Japan. Eur Heart J. 2015; 36:3332–3342.43. Gao R, Yang Y, Han Y, et al. Bioresorbable vascular scaffolds versus metallic stents in patients with coronary artery disease: ABSORB China Trial. J Am Coll Cardiol. 2015; 66:2298–2309.44. Campbell PT, Mahmud E, Marshall JJ. Interoperator and intraoperator (in)accuracy of stent selection based on visual estimation. Catheter Cardiovasc Interv. 2015; 86:1177–1183.45. Gomez-Lara J, Diletti R, Brugaletta S, et al. Angiographic maximal luminal diameter and appropriate deployment of the everolimus-eluting bioresorbable vascular scaffold as assessed by optical coherence tomography: an ABSORB cohort B trial sub-study. EuroIntervention. 2012; 8:214–224.46. Pinton FA. Falcão Bd, Mariani J Jr, Kajita LJ, Filho AE, Lemos PA. Accuracy and precision of online quantitative coronary angiography with automatic calibration: a pilot study. Rev Bras Cardiol Invasiva. 2015; 23:58–60.47. Farooq V, Gomez-Lara J, Brugaletta S, et al. Proximal and distal maximal luminal diameters as a guide to appropriate deployment of the ABSORB everolimus-eluting bioresorbable vascular scaffold: a sub-study of the ABSORB Cohort B and the on-going ABSORB EXTEND Single Arm Study. Catheter Cardiovasc Interv. 2012; 79:880–888.48. Goto K, Mintz GS, Litherland C, et al. Lumen measurements from quantitative coronary angiography and IVUS: a PROSPECT Substudy. JACC Cardiovasc Imaging. 2016; 9:1011–1013.49. Kawamoto H, Jabbour RJ, Tanaka A, Latib A, Colombo A. The bioresorbable scaffold: will oversizing affect outcomes? JACC Cardiovasc Interv. 2016; 9:299–300.50. Ishibashi Y, Nakatani S, Sotomi Y, et al. Relation between bioresorbable scaffold sizing using QCA-Dmax and clinical outcomes at 1 year in 1,232 patients from 3 study cohorts (ABSORB Cohort B, ABSORB EXTEND, and ABSORB II). JACC Cardiovasc Interv. 2015; 8:1715–1726.51. Souteyrand G, Amabile N, Mangin L, et al. Mechanisms of stent thrombosis analysed by optical coherence tomography: insights from the national PESTO French registry. Eur Heart J. 2016; 37:1208–1216.52. Serruys PW, Katagiri Y, Sotomi Y, et al. Arterial remodeling after bioresorbable scaffolds and metallic stents. J Am Coll Cardiol. 2017; 70:60–74.53. Sorrentino S, De Rosa S, Ambrosio G, et al. The duration of balloon inflation affects the luminal diameter of coronary segments after bioresorbable vascular scaffolds deployment. BMC Cardiovasc Disord. 2015; 15:169.54. Elwany M, Latini RA, Di Palma G, Orrego PS, Cortese B. First experience of drug-coated balloons for treatment of bioresorbable vascular scaffold restenosis. [Epub ahead of print]. Cardiovasc Revasc Med. 2017.55. Felix C, Everaert B, Jepson N, Tamburino C, van Geuns RJ. Treatment of bioresorbable scaffold failure. EuroIntervention. 2015; 11:Suppl V. V175–V180.56. Tanaka A, Ruparelia N, Kawamoto H, et al. Clinical outcomes following target lesion revascularization for bioresorbable scaffold failure. Catheter Cardiovasc Interv. 2016; 87:832–836.57. Tanaka A, Jabbour RJ, Mitomo S, Latib A, Colombo A. Hybrid percutaneous coronary intervention with bioresorbable vascular scaffolds in combination with drug-eluting stents or drug-coated balloons for complex coronary lesions. JACC Cardiovasc Interv. 2017; 10:539–547.58. Ielasi A, Miyazaki T, Geraci S, et al. Hybrid strategy with a bioresorbable scaffold and a drug-coated balloon for diffuse coronary artery disease: the “no more metallic cages” multicentre pilot experience. EuroIntervention. 2016; 11:e1589–e1595.59. Levine GN, Bates ER, Blankenship JC, et al. 2015 ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST-elevation myocardial infarction: an update of the 2011 ACCF/AHA/SCAI Guideline for percutaneous coronary intervention and the 2013 ACCF/AHA Guideline for the management of ST-elevation myocardial infarction. J Am Coll Cardiol. 2016; 67:1235–1250.60. Authors/Task Force members. 2014 ESC/EACTS Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014; 35:2541–2619.61. Karanasos A, van Geuns RJ, Zijlstra F, Regar E. Very late bioresorbable scaffold thrombosis after discontinuation of dual antiplatelet therapy. Eur Heart J. 2014; 35:1781.62. Capodanno D, Angiolillo DJ. Antiplatelet therapy after implantation of bioresorbable vascular scaffolds: a review of the published data, practical recommendations, and future directions. JACC Cardiovasc Interv. 2017; 10:425–437.63. Valgimigli M, Bueno H, Byrne RA, et al. ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). [Epub ahead of print]. Eur Heart J. 2017; 2017.64. Puricel S, Arroyo D, Corpataux N, et al. Comparison of everolimus- and biolimus-eluting coronary stents with everolimus-eluting bioresorbable vascular scaffolds. J Am Coll Cardiol. 2015; 65:791–801.65. Sabaté M, Windecker S, Iñiguez A, et al. Everolimus-eluting bioresorbable stent vs. durable polymer everolimus-eluting metallic stent in patients with ST-segment elevation myocardial infarction: results of the randomized ABSORB ST-segment elevation myocardial infarction-TROFI II trial. Eur Heart J. 2016; 37:229–240.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Usefulness of Fibrin Glue in Transantral Orbital Floor Fracture Repair Using Bioresorbable Panel
- Bioresorbable Scaffolds in Coronary Intervention: Unmet Needs and Evolution
- Optical Coherence Tomography and Stent Boost Imaging Guided Bioresorbable Vascular Scaffold Overlapping for Coronary Chronic Total Occlusion Lesion
- Additional fixation using a metal plate with bioresorbable screws and wires for robinson type 2B clavicle fracture
- Scaffold Engineering with Flavone-Modified Biomimetic Architecture for Vascular Tissue Engineering Applications