Restor Dent Endod.
2015 Aug;40(3):223-228. 10.5395/rde.2015.40.3.223.
Changes in SIRT gene expression during odontoblastic differentiation of human dental pulp cells
- Affiliations
-
- 1Department of Conservative Dentistry, Chonnam National University School of Dentistry and Dental Science Research Institute, Gwangju, Korea. ychwang@chonnam.ac.kr
- 2Research Center for Biomineralization Disorders, Chonnam National University, Gwangju, Korea.
- KMID: 2396466
- DOI: http://doi.org/10.5395/rde.2015.40.3.223
Abstract
OBJECTIVES
The aim of this study was to investigate the expression of 7 different sirtuin genes (SIRT1-SIRT7) in human dental pulp cells (HDPCs), and to determine the role of SIRTs in the odontoblastic differentiation potential of HDPCs.
MATERIALS AND METHODS
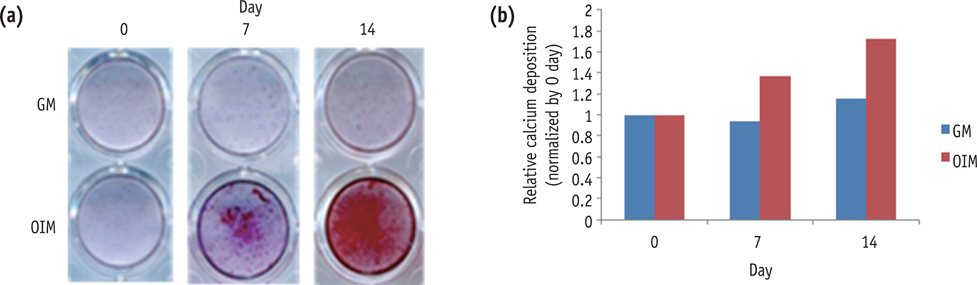
HDPCs were isolated from freshly extracted third molar teeth of healthy patients and cultulred in odontoblastic differentiation inducing media. Osteocalcin (OCN) and dentin sialophosphoprotein (DSPP) expression was analyzed to evaluate the odontoblastic differentiation of HDPCs by reverse transcription-polymerase chain reaction (RT-PCR), while alizarin red staining was used for the mineralization assay. To investigate the expression of SIRTs during odontoblastic differentiation of HDPCs, real time PCR was also performed with RT-PCR.
RESULTS
During the culture of HDPCs in the differentiation inducing media, OCN, and DSPP mRNA expressions were increased. Mineralized nodule formation was also increased in the 14 days culture. All seven SIRT genes were expressed during the odontogenic induction period. SIRT4 expression was increased in a time-dependent manner.
CONCLUSIONS
Our study identified the expression of seven different SIRT genes in HDPCs, and revealed that SIRT4 could exert an influence on the odontoblast differentiation process. Further studies are needed to determine the effects of other SIRTs on the odontogenic potential of HDPCs.
Keyword
MeSH Terms
Figure
Reference
-
1. Fouad AF, Levin L. Pulpal reactions to caries and dental procedures. In : Hargreaves KM, Cohen S, editors. Pathways of the pulp. 10th ed. St. Louis, MO: Mosby Elsevier;2011. p. 504–528.2. Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000; 403:795–800.
Article3. Michishita E, Park JY, Burneskis JM, Barrett JC, Horikawa I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol Biol Cell. 2005; 16:4623–4635.
Article4. Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol. 2010; 5:253–295.
Article5. Lee YM, Shin SI, Shin KS, Lee YR, Park BH, Kim EC. The role of sirtuin 1 in osteoblastic differentiation in human periodontal ligament cells. J Periodontal Res. 2011; 46:712–721.
Article6. Kim JJ, Kim SJ, Kim YS, Kim SY, Park SH, Kim EC. The role of SIRT1 on angiogenic and odontogenic potential in human dental pulp cells. J Endod. 2012; 38:899–906.
Article7. Kitada M, Kume S, Takeda-Watanabe A, Kanasaki K, Koya D. Sirtuins and renal diseases: relationship with aging and diabetic nephropathy. Clin Sci (Lond). 2013; 124:153–164.
Article8. Moschen AR, Wieser V, Gerner RR, Bichler A, Enrich B, Moser P, Ebenbichler CF, Kaser S, Tilg H. Adipose tissue and liver expression of SIRT1, 3, and 6 increase after extensive weight loss in morbid obesity. J Hepatol. 2013; 59:1315–1322.
Article9. Li Z, Xie QR, Chen Z, Lu S, Xia W. Regulation of SIRT2 levels for human non-small cell lung cancer therapy. Lung Cancer. 2013; 82:9–15.
Article10. Sun HL, Wu YR, Huang C, Wang JW, Fu DJ, Liu YC. The effect of SIRT6 on the odontoblastic potential of human dental pulp cells. J Endod. 2014; 40:393–398.
Article11. Wei X, Ling J, Wu L, Liu L, Xiao Y. Expression of mineralization markers in dental pulp cells. J Endod. 2007; 33:703–708.
Article12. Morris BJ. Seven sirtuins for seven deadly diseases of aging. Free Radic Biol Med. 2013; 56:133–171.13. Guarente L. Calorie restriction and sirtuins revisited. Genes Dev. 2013; 27:2072–2085.
Article14. Verdin E, Hirschey MD, Finley LW, Haigis MC. Sirtuin regulation of mitochondria: energy production, apoptosis, and signaling. Trends Biochem Sci. 2010; 35:669–675.
Article15. Nasrin N, Wu X, Fortier E, Feng Y, Bare' OC, Chen S, Ren X, Wu Z, Streeper RS, Bordone L. SIRT4 regulates fatty acid oxidation and mitochondrial gene expression in liver and muscle cells. J Biol Chem. 2010; 285:31995–32002.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- MiR-148a-3p Regulates the Invasion and Odontoblastic Differentiation of Human Dental Pulp Stem Cells via the Wnt1/β-Catenin Pathway
- The Role of Autonomous Wntless in Odontoblastic Differentiation of Mouse Dental Pulp Cells
- Dlx3 and Dlx5 Inhibit Adipogenic Differentiation of Human Dental Pulp Stem Cells
- Analysis of gene expression during odontogenic differentiation of cultured human dental pulp cells
- Comparison of Gene Expression from Supernumerary Dental Pulp and Periodontal Ligament Stem Cells