Tuberc Respir Dis.
2017 Jan;80(1):52-59. 10.4046/trd.2017.80.1.52.
Safety and Effectiveness of Indacaterol in Chronic Obstructive Pulmonary Disease Patients in South Korea
- Affiliations
-
- 1Department of Internal Medicine, Inje University Seoul Paik Hospital, Inje University College of Medicine, Seoul, Korea.
- 2Department of Internal Medicine, Wonkwang University School of Medicine, Iksan, Korea.
- 3Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 4Division of Pulmonology and Allergy, Department of Internal Medicine, Regional Center for Respiratory Disease, Yeungnam University College of Medicine, Daegu, Korea.
- 5Novartis Korea Ltd., Seoul, Korea.
- 6Department of Pulmonary and Critical Care Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. ymoh55@amc.seoul.kr
- KMID: 2396341
- DOI: http://doi.org/10.4046/trd.2017.80.1.52
Abstract
- BACKGROUND
Inhaled indacaterol (Onbrez Breezhaler), a long-acting β₂-agonist, is approved in over 100 countries, including South Korea, as a once-daily bronchodilator for maintenance and treatment of chronic obstructive pulmonary disease (COPD). Here, we present an interim analysis of a post-marketing surveillance study conducted to evaluate the real-world safety and effectiveness of indacaterol in the Korean population.
METHODS
This was an open-label, observational, prospective study in which COPD patients, who were newly prescribed with indacaterol (150 or 300 µg), were evaluated for 12 or 24 weeks. Safety was assessed based on the incidence rates of adverse events (AEs) and serious adverse events (SAEs). Effectiveness was evaluated based on physician's assessment by considering changes in symptoms and lung function, if the values of forced expiratory volume in 1 second were available.
RESULTS
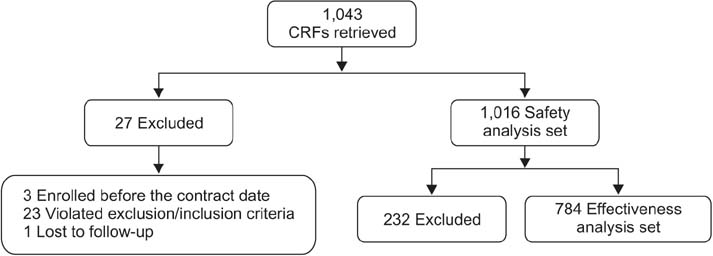
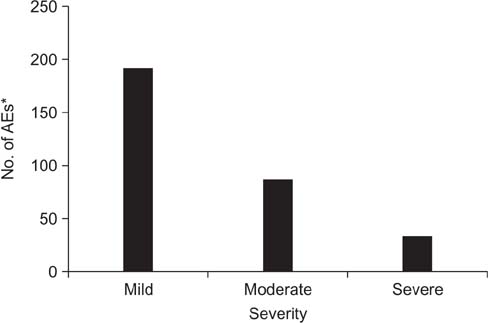
Safety data were analyzed in 1,016 patients of the 1,043 enrolled COPD patients receiving indacaterol, and 784 patients were included for the effectiveness analysis. AEs were reported in 228 (22.44%) patients, while 98 (9.65%) patients reported SAEs. The COPD condition improved in 348 patients (44.4%), while the condition was maintained in 396 patients (50.5%), and only 40 patients (5.1%) exhibited worsening of ailment as compared with baseline. During the treatment period, 90 patients were hospitalized while nine patients died. All deaths were assessed to be not related to the study drug by the investigator.
CONCLUSION
In real-life clinical practice in South Korea, indacaterol was well tolerated in COPD patients, and can be regarded as an effective option for their maintenance treatment.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Pharmacotherapy for chronic obstructive pulmonary disease
In Ae Kim, Yong Bum Park, Kwang Ha Yoo
J Korean Med Assoc. 2018;61(9):545-551. doi: 10.5124/jkma.2018.61.9.545.
Reference
-
1. Gold Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease [Internet]. Gold Initiative for Chronic Obstructive Lung Disease;2016. cited 2016 Jan 12. Available from: http://www.goldcopd.org.2. Park H, Jung SY, Lee K, Bae WK, Lee K, Han JS, et al. Prevalence of chronic obstructive lung disease in Korea using data from the fifth Korea national health and nutrition examination survey. Korean J Fam Med. 2015; 36:128–134.3. Murphy L, Rennard S, Donohue J, Molimard M, Dahl R, Beeh KM, et al. Turning a molecule into a medicine: the development of indacaterol as a novel once-daily bronchodilator treatment for patients with COPD. Drugs. 2014; 74:1635–1657.4. McKeage K. Indacaterol: a review of its use as maintenance therapy in patients with chronic obstructive pulmonary disease. Drugs. 2012; 72:543–563.5. Patalano F, Banerji D, D'Andrea P, Fogel R, Altman P, Colthorpe P. Addressing unmet needs in the treatment of COPD. Eur Respir Rev. 2014; 23:333–344.6. Geake JB, Dabscheck EJ, Wood-Baker R, Cates CJ. Indacaterol, a once-daily beta2-agonist, versus twice-daily beta(2)-agonists or placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015; 1:CD010139.7. Kinoshita M, Lee SH, Hang LW, Ichinose M, Hosoe M, Okino N, et al. Efficacy and safety of indacaterol 150 and 300 microg in chronic obstructive pulmonary disease patients from six Asian areas including Japan: a 12-week, placebo-controlled study. Respirology. 2012; 17:379–389.8. To Y, Kinoshita M, Lee SH, Hang LW, Ichinose M, Fukuchi Y, et al. Assessing efficacy of indacaterol in moderate and severe COPD patients: a 12-week study in an Asian population. Respir Med. 2012; 106:1715–1721.9. Donohue JF, Singh D, Kornmann O, Lawrence D, Lassen C, Kramer B. Safety of indacaterol in the treatment of patients with COPD. Int J Chron Obstruct Pulmon Dis. 2011; 6:477–492.10. Chapman KR, Rennard SI, Dogra A, Owen R, Lassen C, Kramer B, et al. Long-term safety and efficacy of indacaterol, a long-acting beta(2)-agonist, in subjects with COPD: a randomized, placebo-controlled study. Chest. 2011; 140:68–75.11. Ohno T, Wada S, Hanada S, Sawaguchi H, Muraki M, Tohda Y. Efficacy of indacaterol on quality of life and pulmonary function in patients with COPD and inhaler device preferences. Int J Chron Obstruct Pulmon Dis. 2014; 9:107–114.12. Juvelekian G, El-Sorougi W, Pothirat C, Yunus F, De Guia T, Kuo HP, et al. A real-world evaluation of indacaterol and other bronchodilators in COPD: the INFLOW study. Int J Chron Obstruct Pulmon Dis. 2015; 10:2109–2120.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Factors Associated with Indacaterol Response in Tuberculosis-Destroyed Lung with Airflow Limitation
- Effect of Indacaterol on Cough and Phlegm in Chronic Obstructive Pulmonary Disease Patients: A Meta-Analysis of Five Randomized Controlled Trials
- Cor Pulmonale with Particular Reference to Chronic Obstructive Pulmonary Disease and Pulmonary Tuberculosis
- The Clinical Study of Clarithromycin for the Treatment of Acute Exacerbation of Chronic Obstructive Pulmonary Disease
- Management of Chronic Obstructive Pulmonary Disease