Yonsei Med J.
2008 Feb;49(1):144-150.
Intratracheal Administration of Endotoxin Attenuates Hyperoxia-Induced Lung Injury in Neonatal Rats
- Affiliations
-
- 1Department of Pediatrics, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea. wspark@smc.samsung.co.kr
- 2Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
Abstract
- PURPOSE
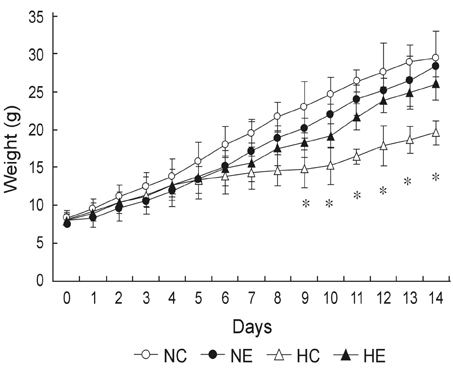
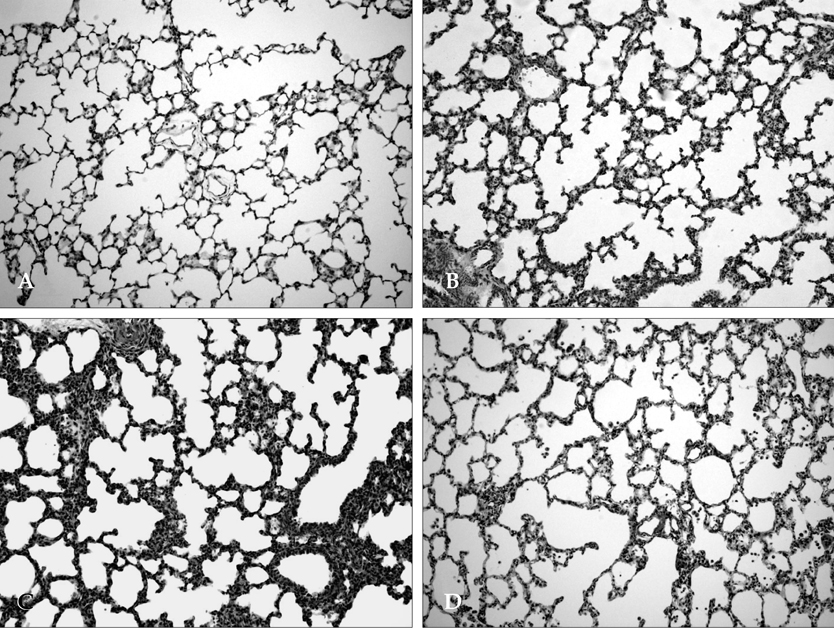
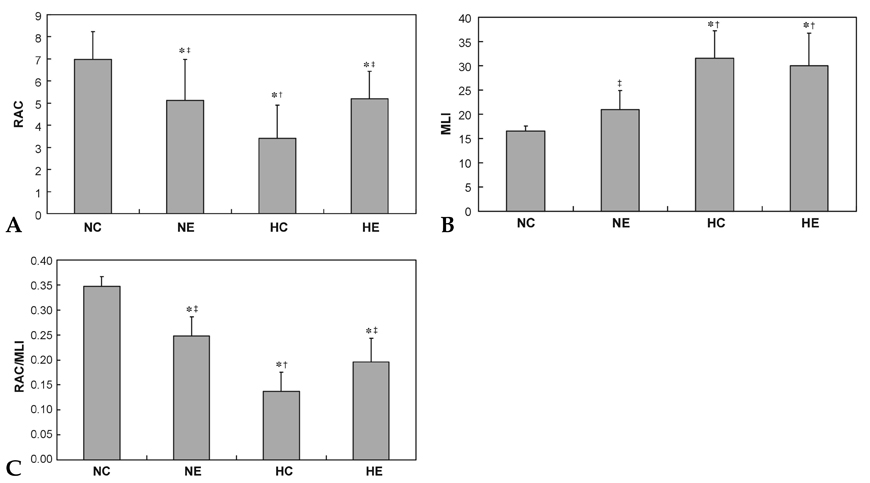
This study was undertaken to determine the effects of intratracheal administration of endotoxin on hyperoxia-induced lung injury in neonatal rats. MATERIALS AND METHODS: Newborn Sprague Dawley rat pups were divided into four experimental groups: normoxia control (NC), normoxia with endotoxin treatment (NE), hyperoxia control (HC), and hyperoxia with endotoxin treatment (HE) groups. In HC and HE, rat pups were subjected to 14 days of hyperoxia (> 95% oxygen) within 12 hours after birth. In endotoxin treated group (NE and HE), Escherichia coli endotoxin (0.5microgram in 0.03mL of saline) was given intratracheally at the 1st, 3rd and 5th postnatal day. Radial alveolar count (RAC), mean linear intercept (MLI), RAC/MLI ratios, and degree of fibrosis were measured to assess the changes in lung morphology. RESULTS: During the research period, survival rates in both HC and HE were notably reduced 7 days after endotoxin was administered, but body weight gain was considerably reduced only in HC. On day 14, significant arrest in alveolarization, as evidenced by the decrease of RAC and RAC/MLI ratio and increase of MLI as well as increased fibrosis, were noted in HC. Although slight but significant arrest in alveolarization and increased fibrosis score were observed in NE compared to NC, the hyperoxia-induced lung damage observed in HC was significantly improved in HE. CONCLUSION: This study suggests that intratracheal administration of endotoxin significantly attenuated hyperoxia-induced lung injury in neonatal rats.
Keyword
MeSH Terms
Figure
Reference
-
1. Abman SH, Groothius JR. Pathophysiology and treatment of bronchopulmonary dysplasia. Current issues. Pediatr Clin North Am. 1994. 41:277–315.
Article2. Saugstad OD. Bronchopulmonary dysplasia-oxidative stress and antioxidants. Semin Neonatol. 2003. 8:39–49.
Article3. Ghezzi F, Gomez R, Romero R, Yoon BH, Edwin SS, David C, et al. Elevated interleukin-8 concentrations in amniotic fluid of mothers whose neonates subsequently develop bronchopulmonary dysplasia. Eur J Obstet Gynecol Reprod Biol. 1998. 78:5–10.
Article4. Jobe AH, Ikegami M. Mechanisms initiating lung injury in the preterm. Early Hum Dev. 1998. 53:81–94.
Article5. Kallapur SG, Jobe AH. Contribution of inflammation to lung injury and development. Arch Dis Child Fetal Neonatal Ed. 2006. 91:F132–F135.
Article6. Rojas MA, Gonzalez A, Bancalari E, Claure N, Poole C, Silva-Neto G. Changing trends in the epidemiology and pathogenesis of neonatal chronic lung disease. J Pediatr. 1995. 126:605–610.
Article7. Watterberg KL, Demers LM, Scott SM, Murphy S. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics. 1996. 97:210–215.
Article8. Costeloe K, Hennessy E, Gibson AT, Marlow N, Wilkinson AR. The EPICure study: outcomes to discharge from hospital for infants born at the threshold of viability. Pediatrics. 2000. 106:659–671.
Article9. Kramer BW, Kramer S, Ikegami M, Jobe AH. Injury, inflammation, and remodeling in fetal sheep lung after intra-amniotic endotoxin. Am J Physiol Lung Cell Mol Physiol. 2002. 283:L452–L459.10. Ueda K, Cho K, Matsuda T, Okajima S, Uchida M, Kobayashi Y, et al. A rat model for arrest of alveolarization induced by antenatal endotoxin administration. Pediatr Res. 2006. 59:396–400.
Article11. Franco ML, Waszak P, Banalec G, Levame M, Lafuma C, Harf A, et al. LPS-induced lung injury in neonatal rats: changes in gelatinase activities and consequences on lung growth. Am J Physiol Lung Cell Mol Physiol. 2002. 282:L491–L500.
Article12. Moss TJ, Newnham JP, Willett KE, Kramer BW, Jobe AH, Ikegami M. Early gestational intra-amniotic endotoxin: lung function, surfactant, and morphometry. Am J Respir Crit Care Med. 2002. 165:805–811.13. Frank L. Oxygen toxicity in neonatal rats: the effect of endotoxin treatment on survival during and post-O2 exposure. Pediatr Res. 1987. 21:109–115.
Article14. Cooney TP, Thurlbeck WM. The radial alveolar count method of Emery and Mithal: a reappraisal 1-postnatal lung growth. Thorax. 1982. 37:572–579.
Article15. Cooney TP, Thurlbeck WM. The radial alveolar count method of Emery and Mithal: a reappraisal 2-intrauterine and early postnatal lung growth. Thorax. 1982. 37:580–583.
Article16. Thurlbeck WM. Measurement of pulmonary emphysema. Am Rev Respir Dis. 1967. 95:752–764.17. Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol. 1988. 41:467–470.
Article18. Massaro D, Teich N, Maxwell S, Massaro GD, Whitney P. Postnatal development of alveoli. Regulation and evidence for a critical period in rats. J Clin Invest. 1985. 76:1297–1305.
Article19. Massaro D, Massaro GD. Dexamethasone accelerates postnatal alveolar wall thinning and alters wall composition. Am J Physiol. 1986. 251:R218–R224.
Article20. Husain AN, Siddiqui NH, Stocker JT. Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum Pathol. 1998. 29:710–717.
Article21. Bonikos DS, Bensch KG, Northway WH Jr. Oxygen toxicity in the newborn. The effect of chronic continuous 100 percent oxygen exposure on the lungs of newborn mice. Am J Pathol. 1976. 85:623–650.22. Wilborn AM, Evers LB, Canada AT. Oxygen toxicity to the developing lung of the mouse: role of reactive oxygen species. Pediatr Res. 1996. 40:225–232.
Article23. Coalson JJ, Winter VT, Siler-Khodr T, Yoder BA. Neonatal chronic lung disease in extremely immature baboons. Am J Respir Crit Care Med. 1999. 160:1333–1346.
Article24. Coalson JJ. Pathology of new bronchopulmonary dysplasia. Semin Neonatol. 2003. 8:73–81.
Article25. Moss TJ, Newnham JP, Willett KE, Kramer BW, Jobe AH, Ikegami M. Early gestational intra-amniotic endotoxin: lung function, surfactant, and morphometry. Am J Respir Crit Care Med. 2002. 165:805–811.26. Kallapur SG, Moss TJ, Ikegami M, Jasman RL, Newnham JP, Jobe AH. Recruited inflammatory cells mediate endotoxin-induced lung maturation in preterm fetal lambs. Am J Respir Crit Care Med. 2005. 172:1315–1321.
Article27. Sosenko IR, Kallapur SG, Nitsos I, Moss TJ, Newnham JP, Ikegami M, et al. IL-1 alpha causes lung inflammation and maturation by direct effects on preterm fetal lamb lungs. Pediatr Res. 2006. 60:294–298.
Article28. Sosenko IR, Jobe AH. Intraamniotic endotoxin increases lung antioxidant enzyme activity in preterm lambs. Pediatr Res. 2003. 53:679–683.
Article29. Bachurski CJ, Ross GF, Ikegami M, Kramer BW, Jobe AH. Intra-amniotic endotoxin increases pulmonary surfactant proteins and induces SP-B processing in fetal sheep. Am J Physiol Lung Cell Mol Physiol. 2001. 280:L279–L285.
Article30. Kallapur SG, Willet KE, Jobe AH, Ikegami M, Bachurski CJ. Intra-amniotic endotoxin: chorioamnionitis precedes lung maturation in preterm lambs. Am J Physiol Lung Cell Mol Physiol. 2001. 280:L527–L536.
Article31. Pillow JJ, Jobe AH, Collins RA, Hantos Z, Ikegami M, Moss TJ, et al. Variability in preterm lamb lung mechanics after intra-amniotic endotoxin is associated with changes in surfactant pool size and morphometry. Am J Physiol Lung Cell Mol Physiol. 2004. 287:L992–L998.
Article32. Keeney SE, Mathews MJ, Shattuck KE, Dallas DV. Endotoxin protection from oxygen toxicity: effect on pulmonary neutrophils and L-selectin. Inflammation. 2002. 26:243–252.33. Bhandari V, Elias JA. Cytokines in tolerance to hyperoxia-induced injury in the developing and adult lung. Free Radic Biol Med. 2006. 41:4–18.
Article34. Kohno M, Ishizaka A, Sawafuji M, Koh H, Hirayama Y, Ikeda E, et al. Hyperoxia-induced emphysematous changes in subacute phase of endotoxin-induced lung injury in rats. Am J Physiol Lung Cell Mol Physiol. 2004. 287:L184–L190.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cell Division Cycle 2 Protects Neonatal Rats Against Hyperoxia-Induced Bronchopulmonary Dysplasia
- Fetal Alveolar Type II Cell Injury Induced by Short-term Exposure to Hyperoxia
- Erythropoietin Attenuates Hyperoxia-Induced Lung Injury by Down-modulating Inflammation in Neonatal Rats
- Development of Lung Injury and Change in Hyaluronan of Extracellular Matrix by the Effect of Hyperoxia in Neonatal Rat
- The effect of surfactant therapy for acute lung injury induced by intratracheal endotoxin instillation in rats examination of the lung