Korean J Physiol Pharmacol.
2017 Nov;21(6):687-694. 10.4196/kjpp.2017.21.6.687.
Biphasic augmentation of alpha-adrenergic contraction by plumbagin in rat systemic arteries
- Affiliations
-
- 1Department of Physiology, Seoul National University College of Medicine, Seoul 03080, Korea. sjoonkim@snu.ac.kr
- 2Hypoxic/Ischemic Disease Institute, Seoul National University College of Medicine, Seoul 03080, Korea.
- 3Chung-Ang University Red Cross College of Nursing, Seoul 06974, Korea.
- 4Department of Internal Medicine, Graduate School of Medicine, Dongguk University, Goyang 10326, Korea. wk2kim@naver.com
- 5Channelopathy Research Center (CRC), Dongguk University College of Medicine, Goyang 10326, Korea.
- KMID: 2395264
- DOI: http://doi.org/10.4196/kjpp.2017.21.6.687
Abstract
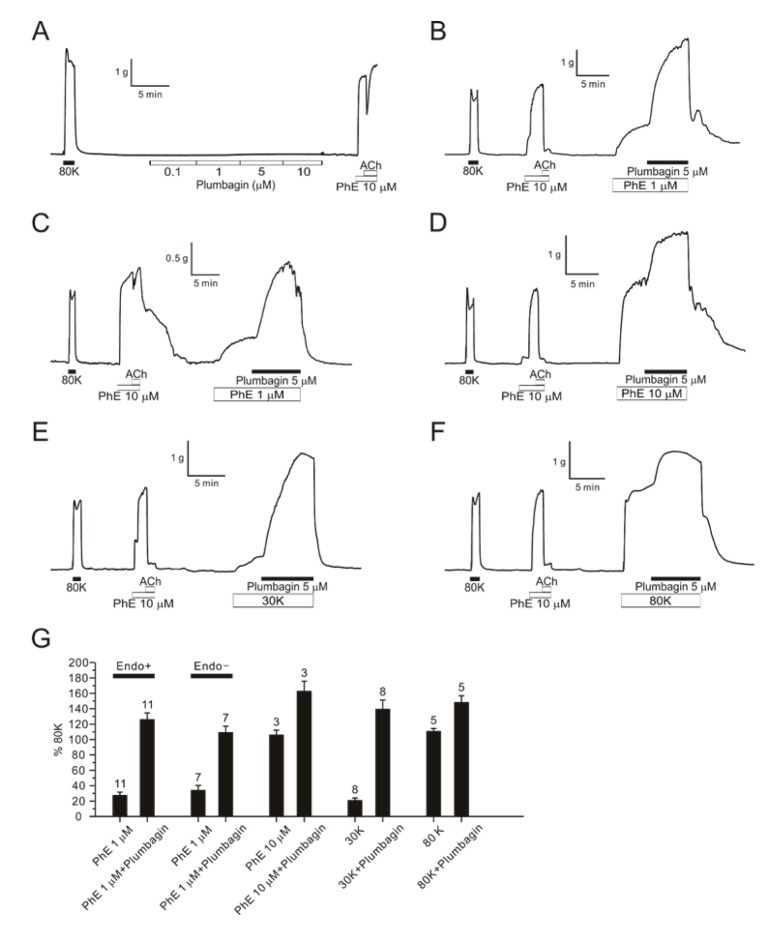
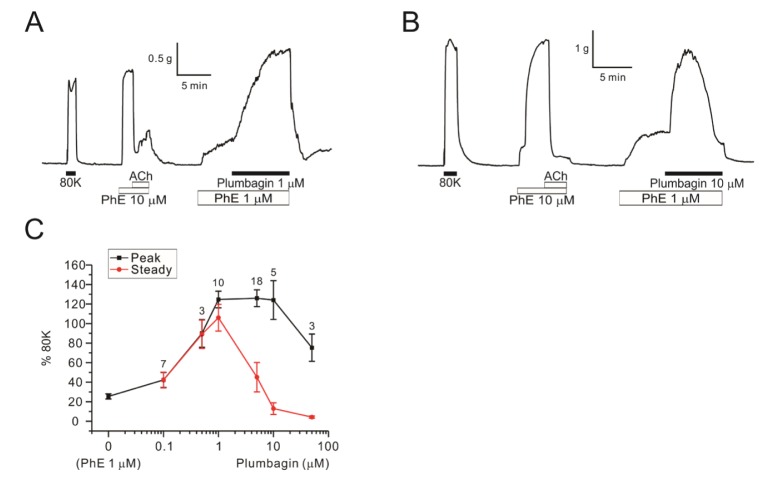
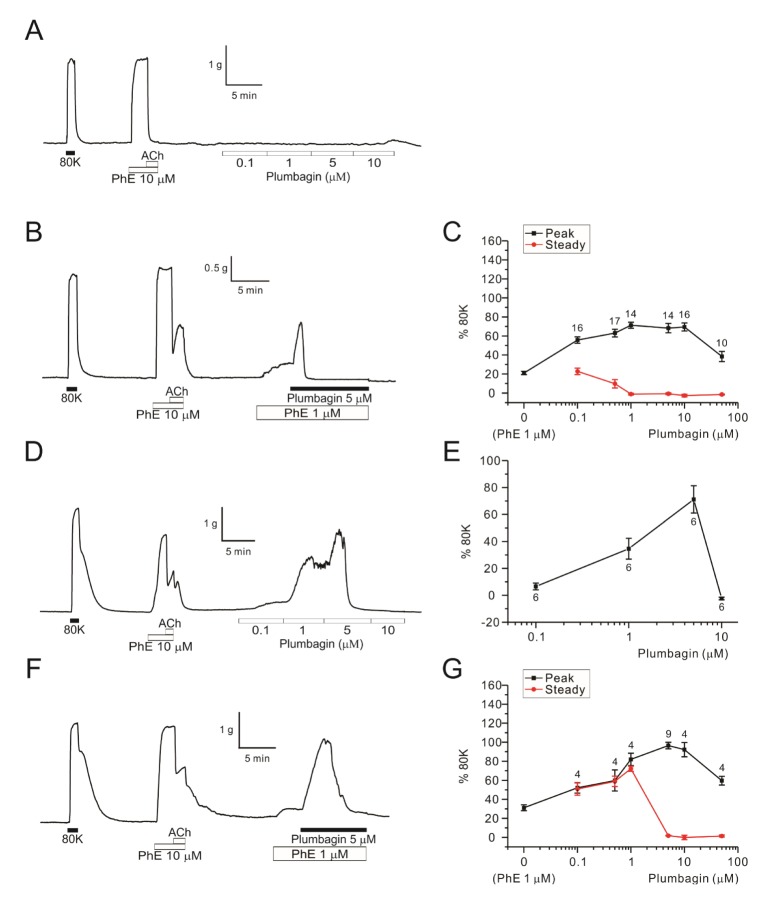
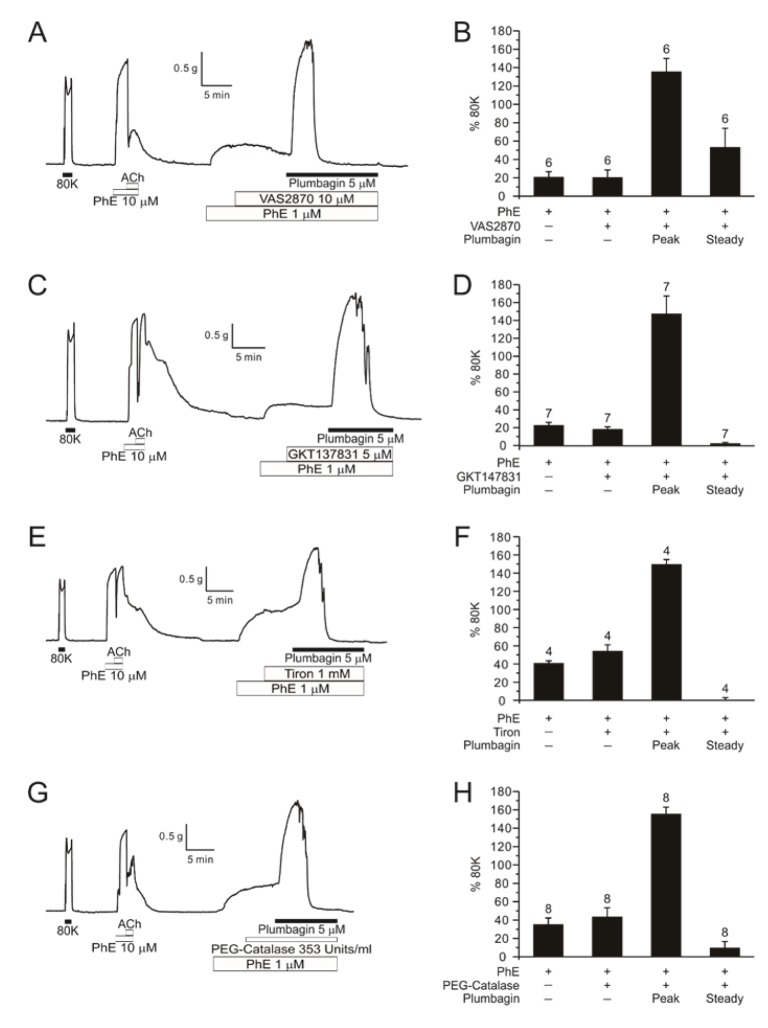
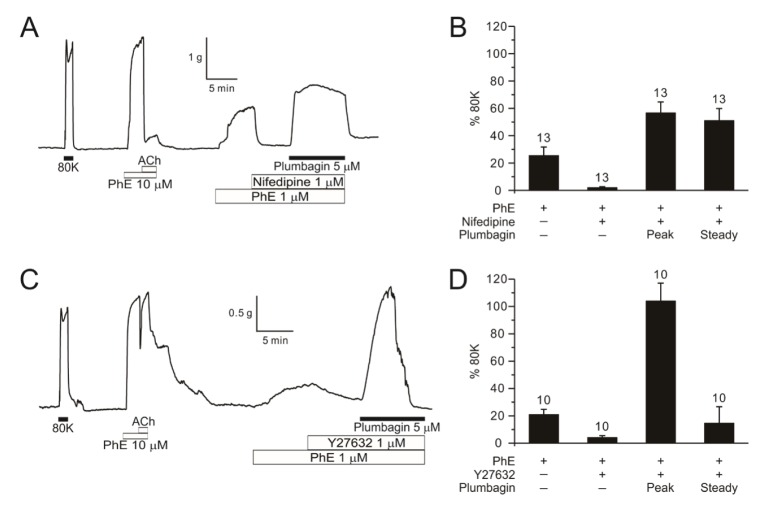
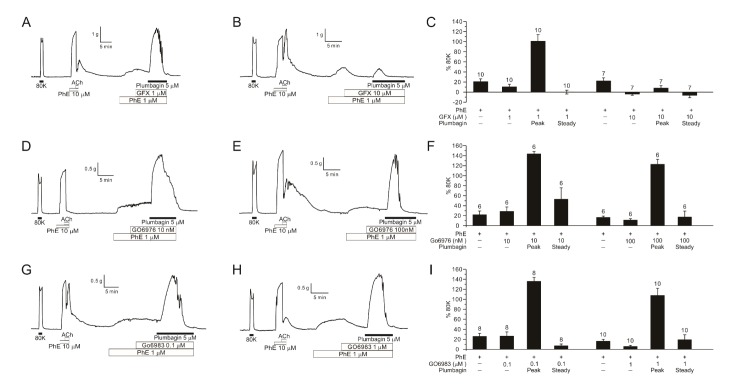
- Plumbagin, a hydroxy 1,4-naphthoquinone compound from plant metabolites, exhibits anticancer, antibacterial, and antifungal activities via modulating various signaling molecules. However, its effects on vascular functions are rarely studied except in pulmonary and coronary arteries where NADPH oxidase (NOX) inhibition was suggested as a mechanism. Here we investigate the effects of plumbagin on the contractility of skeletal artery (deep femoral artery, DFA), mesenteric artery (MA) and renal artery (RA) in rats. Although plumbagin alone had no effect on the isometric tone of DFA, 1 µM phenylephrine (PhE)-induced partial contraction was largely augmented by plumbagin (ΔT(Plum), 125% of 80 mM KCl-induced contraction at 1 µM). With relatively higher concentrations (>5 µM), plumbagin induced a transient contraction followed by tonic relaxation of DFA. Similar biphasic augmentation of the PhE-induced contraction was observed in MA and RA. VAS2870 and GKT137831, specific NOX4 inhibitors, neither mimicked nor inhibited ΔT(Plum) in DFA. Also, pretreatment with tiron or catalase did not affect ΔT(Plum) of DFA. Under the inhibition of PhE-contraction with L-type Ca²âº channel blocker (nifedipine, 1 µM), plumbagin still induced tonic contraction, suggesting Ca²âº-sensitization mechanism of smooth muscle. Although ΔT(Plum) was consistently observed under pretreatment with Rho A-kinase inhibitor (Y27632, 1 µM), a PKC inhibitor (GF 109203X, 10 µM) largely suppressed ΔT(Plum). Taken together, it is suggested that plumbagin facilitates the PKC activation in the presence of vasoactive agonists in skeletal arteries. The biphasic contractile effects on the systemic arteries should be considered in the pharmacological studies of plumbagin and 1,4-naphthoquinones.
Keyword
MeSH Terms
-
1,2-Dihydroxybenzene-3,5-Disulfonic Acid Disodium Salt
Animals
Arteries*
Catalase
Coronary Vessels
Femoral Artery
Mesenteric Arteries
Muscle, Smooth
NADPH Oxidase
Phenylephrine
Plants
Protein Kinase C
Rats*
Relaxation
Renal Artery
Vasoconstrictor Agents
1,2-Dihydroxybenzene-3,5-Disulfonic Acid Disodium Salt
Catalase
NADPH Oxidase
Phenylephrine
Protein Kinase C
Vasoconstrictor Agents
Figure
Reference
-
1. Padhye S, Dandawate P, Yusufi M, Ahmad A, Sarkar FH. Perspectives on medicinal properties of plumbagin and its analogs. Med Res Rev. 2012; 32:1131–1158. PMID: 23059762.
Article2. Courboulin A, Barrier M, Perreault T, Bonnet P, Tremblay VL, Paulin R, Tremblay E, Lambert C, Jacob MH, Bonnet SN, Provencher S, Bonnet S. Plumbagin reverses proliferation and resistance to apoptosis in experimental PAH. Eur Respir J. 2012; 40:618–629. PMID: 22496325.
Article3. Ding Y, Chen ZJ, Liu S, Che D, Vetter M, Chang CH. Inhibition of Nox-4 activity by plumbagin, a plant-derived bioactive naphthoquinone. J Pharm Pharmacol. 2005; 57:111–116. PMID: 15638999.
Article4. Ahmad M, Kelly MR, Zhao X, Kandhi S, Wolin MS. Roles for Nox4 in the contractile response of bovine pulmonary arteries to hypoxia. Am J Physiol Heart Circ Physiol. 2010; 298:H1879–H1888. PMID: 20304813.
Article5. Smith RM, Kruzliak P, Adamcikova Z, Zulli A. Role of Nox inhibitors plumbagin, ML090 and gp91ds-tat peptide on homocysteine thiolactone induced blood vessel dysfunction. Clin Exp Pharmacol Physiol. 2015; 42:860–864. PMID: 25998981.
Article6. Han JA, Seo EY, Kim HJ, Park SJ, Yoo HY, Kim JY, Shin DM, Kim JK, Zhang YH, Kim SJ. Hypoxia-augmented constriction of deep femoral artery mediated by inhibition of eNOS in smooth muscle. Am J Physiol Cell Physiol. 2013; 304:C78–C88. PMID: 23099643.
Article7. Altenhöfer S, Radermacher KA, Kleikers PW, Wingler K, Schmidt HH. Evolution of NADPH oxidase inhibitors: selectivity and mechanisms for target engagement. Antioxid Redox Signal. 2015; 23:406–427. PMID: 24383718.
Article8. Kim JA, Neupane GP, Lee ES, Jeong BS, Park BC, Thapa P. NADPH oxidase inhibitors: a patent review. Expert Opin Ther Pat. 2011; 21:1147–1158. PMID: 21554154.
Article9. Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and non-muscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003; 83:1325–1358. PMID: 14506307.10. Hafeez BB, Zhong W, Mustafa A, Fischer JW, Witkowsky O, Verma AK. Plumbagin inhibits prostate cancer development in TRAMP mice via targeting PKCε, Stat3 and neuroendocrine markers. Carcinogenesis. 2012; 33:2586–2592. PMID: 22976928.
Article11. Hafeez BB, Zhong W, Fischer JW, Mustafa A, Shi X, Meske L, Hong H, Cai W, Havighurst T, Kim K, Verma AK. Plumbagin, a medicinal plant (Plumbago zeylanica)-derived 1,4-naphthoquinone, inhibits growth and metastasis of human prostate cancer PC-3Mluciferase cells in an orthotopic xenograft mouse model. Mol Oncol. 2013; 7:428–439. PMID: 23273564.12. Hafeez BB, Jamal MS, Fischer JW, Mustafa A, Verma AK. Plumbagin, a plant derived natural agent inhibits the growth of pancreatic cancer cells in in vitro and in vivo via targeting EGFR, Stat3 and NF-κB signaling pathways. Int J Cancer. 2012; 131:2175–2186. PMID: 22322442.
Article13. Way KJ, Chou E, King GL. Identification of PKC-isoform-specific biological actions using pharmacological approaches. Trends Pharmacol Sci. 2000; 21:181–187. PMID: 10785652.
Article14. Ringvold HC, Khalil RA. Protein kinase C as regulator of vascular smooth muscle function and potential target in vascular disorders. Adv Pharmacol. 2017; 78:203–301. PMID: 28212798.
Article15. Joo HK, Lee YR, Choi S, Park MS, Kang G, Kim CS, Jeon BH. Protein kinase C beta II upregulates intercellular adhesion molecule-1 via mitochondrial activation in cultured endothelial cells. Korean J Physiol Pharmacol. 2017; 21:377–384. PMID: 28706451.
Article16. Floreani M, Forlin A, Pandolfo L, Petrone M, Bellin S. Mechanisms of plumbagin action on guinea pig isolated atria. J Pharmacol Exp Ther. 1996; 278:763–770. PMID: 8768729.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Differential effects of saturated and unsaturated fatty acids on vascular reactivity in isolated mesenteric and femoral arteries of rats
- The Studies on the Contractile Response of Serotinin in Rat Aorta
- Biphasic anaphylaxis to gemifloxacin
- Responses of the Detrusor Muscle Strips of the Amyda Japonica and the Rabbit to some Autonomic Drugs
- The Role of beta-Adrenergic Receptor in the Seminal Vesicle Contraction