Korean J Physiol Pharmacol.
2019 Sep;23(5):403-409. 10.4196/kjpp.2019.23.5.403.
Differential effects of saturated and unsaturated fatty acids on vascular reactivity in isolated mesenteric and femoral arteries of rats
- Affiliations
-
- 1Graduate School, Chung-Ang University, Seoul 06974, Korea.
- 2Department of Nursing, Chung-Ang University, Seoul 06974, Korea. hyoo@cau.ac.kr
- KMID: 2455816
- DOI: http://doi.org/10.4196/kjpp.2019.23.5.403
Abstract
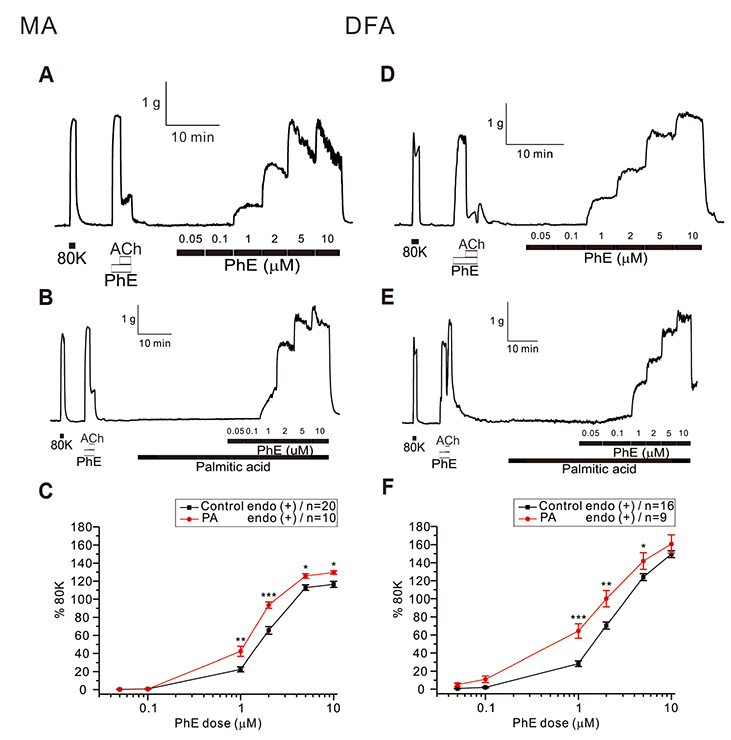
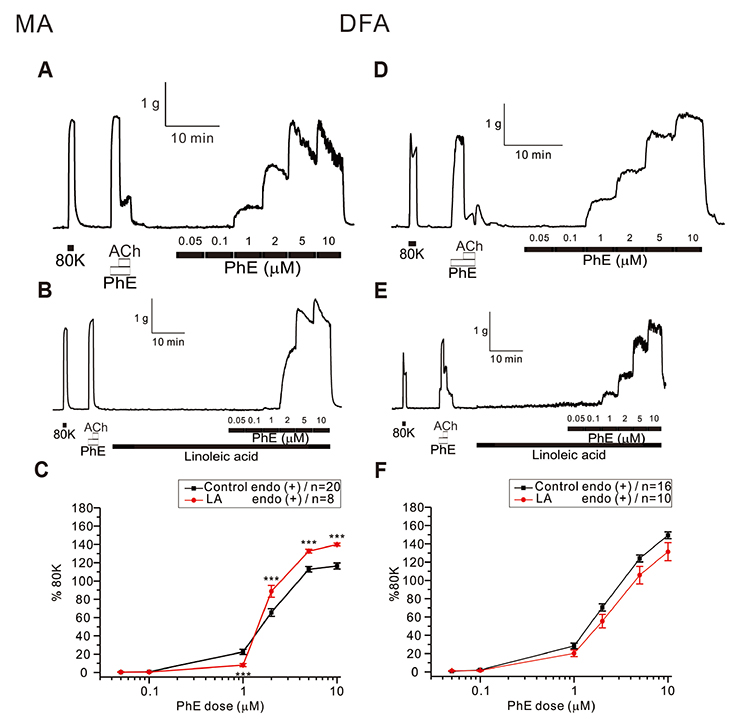
- Free fatty acid (FFA) intake regulates blood pressure and vascular reactivity but its direct effect on contractility of systemic arteries is not well understood. We investigated the effects of saturated fatty acid (SFA, palmitic acid), polyunsaturated fatty acid (PUFA, linoleic acid), and monounsaturated fatty acid (MUFA, oleic acid) on the contractility of isolated mesenteric (MA) and deep femoral arteries (DFA) of Sprague-Dawley rats. Isolated MA and DFA were mounted on a dual wire myograph and phenylephrine (PhE, 1-10 µM) concentration-dependent contraction was obtained with or without FFAs. Incubation with 100 µM of palmitic acid significantly increased PhE-induced contraction in both arteries. In MA, treatment with 100 µM of linoleic acid decreased 1 µM PhE-induced contraction while increasing the response to higher PhE concentrations. In DFA, linoleic acid slightly decreased PhE-induced contraction while 200 µM oleic acid significantly decreased it. In MA, oleic acid reduced contraction at low PhE concentration (1 and 2 µM) while increasing it at 10 µM PhE. Perplexingly, depolarization by 40 mM KCl-induced contraction of MA was commonly enhanced by the three fatty acids. The 40 mM KCl-contraction of DFA was also augmented by linoleic and oleic acids while not affected by palmitic acid. SFA persistently increased alpha-adrenergic contraction of systemic arteries whereas PUFA and MUFA attenuated PhE-induced contraction of skeletal arteries. PUFA and MUFA concentration-dependent dual effects on MA suggest differential mechanisms depending on the types of arteries. Further studies are needed to elucidate underlying mechanisms of the various effects of FFA on systemic arteries.
Keyword
MeSH Terms
-
Animals
Arteries
Blood Pressure
Fatty Acids
Fatty Acids, Unsaturated*
Femoral Artery*
Linoleic Acid
Mesenteric Arteries
Oleic Acid
Oleic Acids
Palmitic Acid
Phenylephrine
Rats*
Receptors, Adrenergic, alpha
Vasoconstriction
Fatty Acids
Fatty Acids, Unsaturated
Linoleic Acid
Oleic Acid
Oleic Acids
Palmitic Acid
Phenylephrine
Receptors, Adrenergic, alpha
Figure
Reference
-
1. Pagidipati NJ, Gaziano TA. Estimating deaths from cardiovascular disease: a review of global methodologies of mortality measurement. Circulation. 2013; 127:749–756.
Article2. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016; 133:e38–e360.3. Gaziano TA. Reducing the growing burden of cardiovascular disease in the developing world. Health Aff (Millwood). 2007; 26:13–24.
Article4. McNaughton SA, Mishra GD, Brunner EJ. Food patterns associated with blood lipids are predictive of coronary heart disease: the Whitehall II study. Br J Nutr. 2009; 102:619–624.
Article5. McNaughton SA, Mishra GD, Brunner EJ. Dietary patterns, insulin resistance, and incidence of type 2 diabetes in the Whitehall II Study. Diabetes Care. 2008; 31:1343–1348.
Article6. Deshmukh-Taskar PR, O'Neil CE, Nicklas TA, Yang SJ, Liu Y, Gustat J, Berenson GS. Dietary patterns associated with metabolic syndrome, sociodemographic and lifestyle factors in young adults: the Bogalusa Heart Study. Public Health Nutr. 2009; 12:2493–2503.
Article7. Münzel T, Gori T, Bruno RM, Taddei S. Is oxidative stress a therapeutic target in cardiovascular disease? Eur Heart J. 2010; 31:2741–2748.
Article8. Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003; 23:168–175.9. Sandoo A, van Zanten JJ, Metsios GS, Carroll D, Kitas GD. The endothelium and its role in regulating vascular tone. Open Cardiovasc Med J. 2010; 4:302–312.
Article10. Hooper L, Bartlett C, Davey SG, Ebrahim S. Advice to reduce dietary salt for prevention of cardiovascular disease. Cochrane Database Syst Rev. 2004; (1):CD003656.
Article11. American Heart Association Nutrition Committee. Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, Franklin B, Kris-Etherton P, Harris WS, Howard B, Karanja N, Lefevre M, Rudel L, Sacks F, Van Horn L, Winston M, Wylie-Rosett J. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006; 114:82–96.12. Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, Lee IM, Lichtenstein AH, Loria CM, Millen BE, Nonas CA, Sacks FM, Smith SC Jr, Svetkey LP, Wadden TA, Yanovski SZ, Kendall KA, Morgan LC, Trisolini MG, Velasco G, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014; 129:25 Suppl 2. S76–S99.13. DiNicolantonio JJ, Lucan SC, O'Keefe JH. The evidence for saturated fat and for sugar related to coronary heart disease. Prog Cardiovasc Dis. 2016; 58:464–472.
Article14. Hooper L, Martin N, Abdelhamid A, Davey Smith G. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst Rev. 2015; (6):CD011737.
Article15. Kris-Etherton PM, Fleming JA. Emerging nutrition science on fatty acids and cardiovascular disease: nutritionists’ perspectives. Adv Nutr. 2015; 6:326S–337S.
Article16. Vafeiadou K, Weech M, Altowaijri H, Todd S, Yaqoob P, Jackson KG, Lovegrove JA. Replacement of saturated with unsaturated fats had no impact on vascular function but beneficial effects on lipid biomarkers, E-selectin, and blood pressure: results from the randomized, controlled Dietary Intervention and VAScular function (DIVAS) study. Am J Clin Nutr. 2015; 102:40–48.
Article17. Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Saturated fatty acids and risk of coronary heart disease: modulation by replacement nutrients. Curr Atheroscler Rep. 2010; 12:384–390.
Article18. Jakobsen MU, O'Reilly EJ, Heitmann BL, Pereira MA, Bälter K, Fraser GE, Goldbourt U, Hallmans G, Knekt P, Liu S, Pietinen P, Spiegelman D, Stevens J, Virtamo J, Willett WC, Ascherio A. Major types of dietary fat and risk of coronary heart disease: a pooled analysis of 11 cohort studies. Am J Clin Nutr. 2009; 89:1425–1432.
Article19. Saraswathi V, Wu G, Toborek M, Hennig B. Linoleic acid-induced endothelial activation: role of calcium and peroxynitrite signaling. J Lipid Res. 2004; 45:794–804.20. Chinen I, Shimabukuro M, Yamakawa K, Higa N, Matsuzaki T, Noguchi K, Ueda S, Sakanashi M, Takasu N. Vascular lipotoxicity: endothelial dysfunction via fatty-acid-induced reactive oxygen species overproduction in obese Zucker diabetic fatty rats. Endocrinology. 2007; 148:160–165.
Article21. Sainsbury CA, Sattar N, Connell JM, Hillier C, Petrie JR. Non-esterified fatty acids impair endothelium-dependent vasodilation in rat mesenteric resistance vessels. Clin Sci (Lond). 2004; 107:625–629.
Article22. Lundman P, Tornvall P, Nilsson L, Pernow J. A triglyceride-rich fat emulsion and free fatty acids but not very low density lipoproteins impair endothelium-dependent vasorelaxation. Atherosclerosis. 2001; 159:35–41.
Article23. Sun X, Hou N, Han F, Guo Y, Hui Z, Du G, Zhang Y. Effect of high free fatty acids on the anti-contractile response of perivascular adipose tissue in rat aorta. J Mol Cell Cardiol. 2013; 63:169–174.
Article24. Steinberg HO, Tarshoby M, Monestel R, Hook G, Cronin J, Johnson A, Bayazeed B, Baron AD. Elevated circulating free fatty acid levels impair endothelium-dependent vasodilation. J Clin Invest. 1997; 100:1230–1239.
Article25. Pleiner J, Schaller G, Mittermayer F, Bayerle-Eder M, Roden M, Wolzt M. FFA-induced endothelial dysfunction can be corrected by vitamin C. J Clin Endocrinol Metab. 2002; 87:2913–2917.
Article26. Nitenberg A, Cosson E, Pham I. Postprandial endothelial dysfunction: role of glucose, lipids and insulin. Diabetes Metab. 2006; 32:2S28–2S33.
Article27. Watanabe S, Tagawa T, Yamakawa K, Shimabukuro M, Ueda S. Inhibition of the renin-angiotensin system prevents free fatty acidinduced acute endothelial dysfunction in humans. Arterioscler Thromb Vasc Biol. 2005; 25:2376–2380.
Article28. Volpe CM, Nogueira-Machado JA. The dual role of free fatty acid signaling in inflammation and therapeutics. Recent Pat Endocr Metab Immune Drug Discov. 2013; 7:189–197.
Article29. Bhagavan NV, Ha JS, Park JH, Honda SA, Rios CN, Sugiyama C, Fujitani GK, Takeuchi IK, Ha CE. Utility of serum Fatty Acid concentrations as a marker for acute myocardial infarction and their potential role in the formation of ischemia-modified albumin: a pilot study. Clin Chem. 2009; 55:1588–1590.
Article30. Doronzo G, Viretto M, Barale C, Russo I, Mattiello L, Anfossi G, Trovati M. Oleic acid increases synthesis and secretion of VEGF in rat vascular smooth muscle cells: role of oxidative stress and impairment in obesity. Int J Mol Sci. 2013; 14:18861–18880.31. Vorn R, Yoo HY. Effects of high glucose with or without other metabolic substrates on alpha-adrenergic contractions in rat mesenteric and femoral arteries. Korean J Physiol Pharmacol. 2017; 21:91–97.
Article32. Harvey KA, Walker CL, Pavlina TM, Xu Z, Zaloga GP, Siddiqui RA. Long-chain saturated fatty acids induce pro-inflammatory responses and impact endothelial cell growth. Clin Nutr. 2010; 29:492–500.
Article33. Djoussé L, Benkeser D, Arnold A, Kizer JR, Zieman SJ, Lemaitre RN, Tracy RP, Gottdiener JS, Mozaffarian D, Siscovick DS, Mukamal KJ, Ix JH. Plasma free fatty acids and risk of heart failure: the Cardiovascular Health Study. Circ Heart Fail. 2013; 6:964–969.34. Gao Z, Zhang H, Liu J, Lau CW, Liu P, Chen ZY, Lee HK, Tipoe GL, Ho HM, Yao X, Huang Y. Cyclooxygenase-2-dependent oxidative stress mediates palmitate-induced impairment of endothelium-dependent relaxations in mouse arteries. Biochem Pharmacol. 2014; 91:474–482.
Article35. Li H, Li H, Bao Y, Zhang X, Yu Y. Free fatty acids induce endothelial dysfunction and activate protein kinase C and nuclear factor-κB pathway in rat aorta. Int J Cardiol. 2011; 152:218–224.
Article36. Tousoulis D, Kampoli AM, Tentolouris C, Papageorgiou N, Stefanadis C. The role of nitric oxide on endothelial function. Curr Vasc Pharmacol. 2012; 10:4–18.
Article37. Ferdinandy P, Schulz R. Nitric oxide, superoxide, and peroxynitrite in myocardial ischaemia-reperfusion injury and preconditioning. Br J Pharmacol. 2003; 138:532–543.
Article38. Engler MB. Effects of omega-3, omega-6 and omega-9 fatty acids on vascular smooth muscle tone. Eur J Pharmacol. 1992; 215:325–328.
Article39. Pomposiello SI, Alva M, Wilde DW, Carretero OA. Linoleic acid induces relaxation and hyperpolarization of the pig coronary artery. Hypertension. 1998; 31:615–620.
Article40. Christon RA. Mechanisms of action of dietary fatty acids in regulating the activation of vascular endothelial cells during atherogenesis. Nutr Rev. 2003; 61:272–279.
Article41. Medei E, Lima-Leopoldo AP, Pereira-Junior PP, Leopoldo AS, Campos DH, Raimundo JM, Sudo RT, Zapata-Sudo G, Bruder-Nascimento T, Cordellini S, Nascimento JH, Cicogna AC. Could a high-fat diet rich in unsaturated fatty acids impair the cardiovascular system? Can J Cardiol. 2010; 26:542–548.
Article42. Perdomo L, Beneit N, Otero YF, Escribano Ó, Díaz-Castroverde S, Gómez-Hernández A, Benito M. Protective role of oleic acid against cardiovascular insulin resistance and in the early and late cellular atherosclerotic process. Cardiovasc Diabetol. 2015; 14:75.
Article43. Gao D, Griffiths HR, Bailey CJ. Oleate protects against palmitate-induced insulin resistance in L6 myotubes. Br J Nutr. 2009; 102:1557–1563.
Article44. Greene EL, Lu G, Zhang D, Egan BM. Signaling events mediating the additive effects of oleic acid and angiotensin II on vascular smooth muscle cell migration. Hypertension. 2001; 37:308–312.
Article45. Marchand A, Abi-Gerges A, Saliba Y, Merlet E, Lompré AM. Calcium signaling in vascular smooth muscle cells: from physiology to pathology. Adv Exp Med Biol. 2012; 740:795–810.
Article46. Liu Z, Khalil RA. Evolving mechanisms of vascular smooth muscle contraction highlight key targets in vascular disease. Biochem Pharmacol. 2018; 153:91–122.
Article47. Yamamura A, Guo Q, Yamamura H, Zimnicka AM, Pohl NM, Smith KA, Fernandez RA, Zeifman A, Makino A, Dong H, Yuan JX. Enhanced Ca2+-sensing receptor function in idiopathic pulmonary arterial hypertension. Circ Res. 2012; 111:469–481.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of high glucose with or without other metabolic substrates on alpha-adrenergic contractions in rat mesenteric and femoral arteries
- Diabetes and Dietary Fats
- Diabetes and Nuts
- Effect of w-3 polyunsaturated fatty acids supplementation diet oninsulin binding, and generation of diabetes in STZ-injected femalerats
- Differential Effects of Palmitate and Docosahexaenoic acid on ATP-sensitive K+ Channel Activity of Pancreatic beta-cells