Ann Dermatol.
2017 Dec;29(6):715-721. 10.5021/ad.2017.29.6.715.
Increased Skin Irritation by Hydroquinone and Rsetinoic Acid Used in Combination
- Affiliations
-
- 1Department of Dermatology, Dongguk University Ilsan Hospital, Goyang, Korea. lay5604@naver.com
- KMID: 2395178
- DOI: http://doi.org/10.5021/ad.2017.29.6.715
Abstract
- BACKGROUND
Hydroquinone (HQ) is frequently combined with retinoic acid (RA) to enhance lightening efficacy, which may also affect skin irritancy. Although skin irritation leads to postinflammatory hyperpigmentation, little research has been performed to compare skin irritancy between each component and the combination.
OBJECTIVE
This study was done to examine whether HQ-RA combination increased skin irritation induced by HQ or RA alone.
METHODS
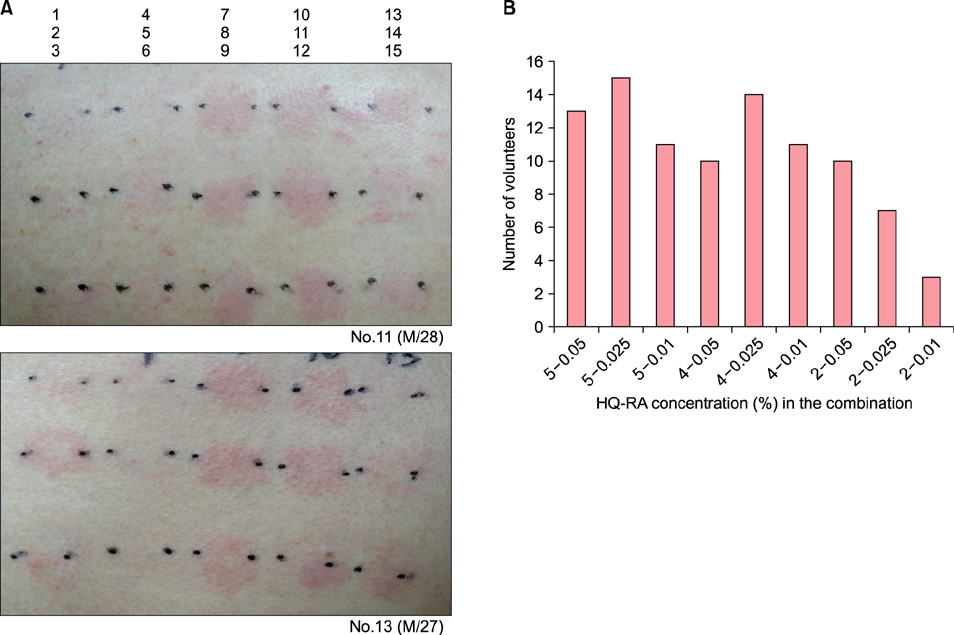
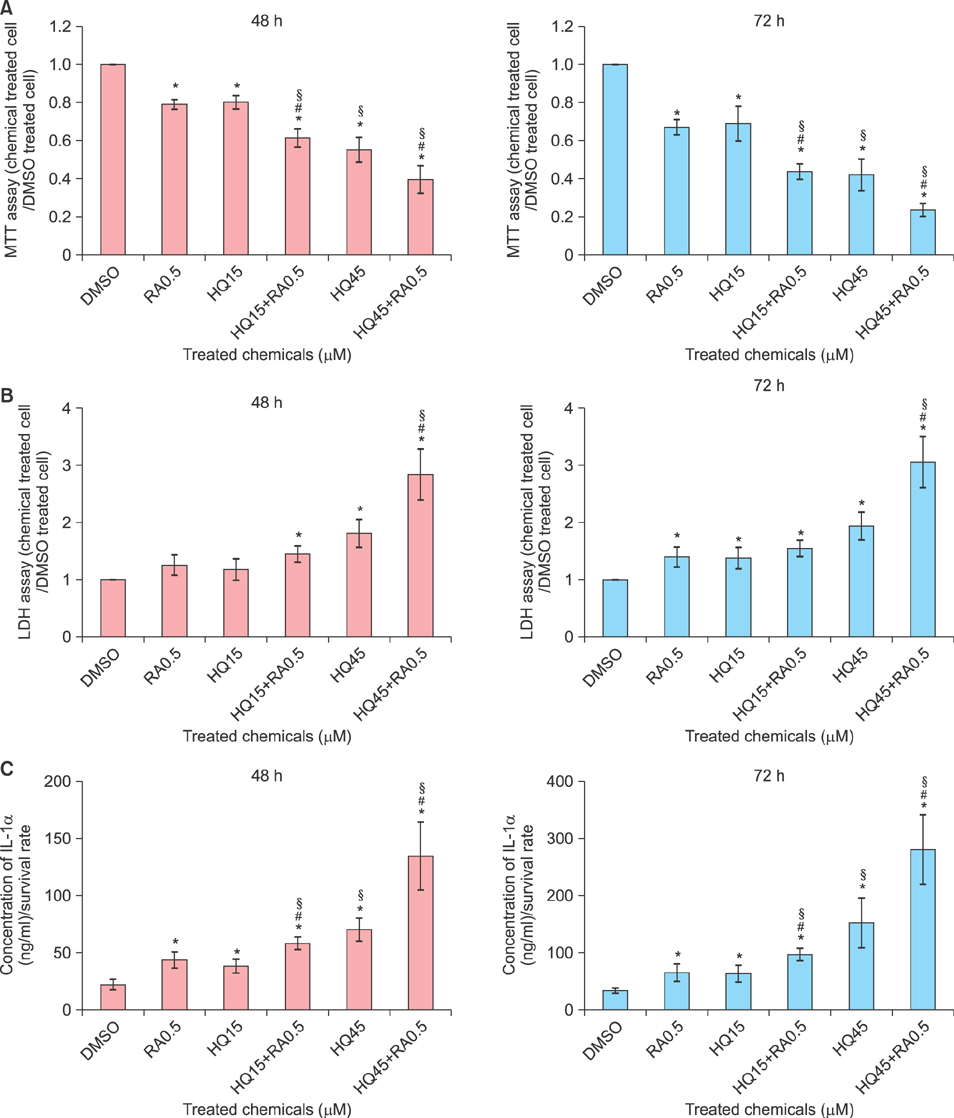
Patch testing was performed using maximum therapeutic and higher concentrations of HQ and RA in 10 volunteers, and then, it was performed using their popular therapeutic concentrations and combination in the other 20 volunteers. In vitro irritation was also assessed in primary cultured normal human keratinocytes treated with 80% and 50% cell survival doses of HQ, 80% cell survival dose of RA, and their combination.
RESULTS
The combination in patch testing induced stronger erythema than the corresponding concentrations of HQ and RA, which was remarkable with use of combination of higher concentrations. In cultured keratinocytes, the RA combination significantly decreased cell viability, but increased cytotoxicity and extracellular interleukin 1 alpha release with corresponding doses of HQ.
CONCLUSION
The results of patch tests and in vitro irritation assessment tests suggested that HQ and RA increased skin irritation when used in combination.
Keyword
MeSH Terms
Figure
Reference
-
1. Matsubayashi T, Sakaeda T, Kita T, Kurimoto Y, Nakamura T, Nishiguchi K, et al. Intradermal concentration of hydroquinone after application of hydroquinone ointments is higher than its cytotoxic concentration. Biol Pharm Bull. 2003; 26:1365–1367.
Article2. Stratigos AJ, Katsambas AD. The role of topical retinoids in the treatment of photoaging. Drugs. 2005; 65:1061–1072.
Article3. Hexsel D, Arellano I, Rendon M. Ethnic considerations in the treatment of Hispanic and Latin-American patients with hyperpigmentation. Br J Dermatol. 2006; 156:Suppl 1. 7–12.
Article4. Ho SG, Chan HH. The Asian dermatologic patient: review of common pigmentary disorders and cutaneous diseases. Am J Clin Dermatol. 2009; 10:153–168.5. van Ruissen F, Le M, Carroll JM, van der Valk PG, Schalkwijk J. Differential effects of detergents on keratinocyte gene expression. J Invest Dermatol. 1998; 110:358–363.
Article6. Fluhr JW, Darlenski R, Angelova-Fischer I, Tsankov N, Basketter D. Skin irritation and sensitization: mechanisms and new approaches for risk assessment. 1. Skin irritation. Skin Pharmacol Physiol. 2008; 21:124–135.
Article7. Ale IS, Maibach HI. Irritant contact dermatitis. Rev Environ Health. 2014; 29:195–206.
Article8. Osborne R, Perkins MA. In vitro skin irritation testing with human skin cell cultures. Toxicol In Vitro. 1991; 5:563–567.
Article9. Korting HC, Schindler S, Hartinger A, Kerscher M, Angerpointner T, Maibach HI. MTT-assay and neutral red release (NRR)-assay: relative role in the prediction of the irritancy potential of surfactants. Life Sci. 1994; 55:533–540.
Article10. Corsini E, Galli CL. Cytokines and irritant contact dermatitis. Toxicol Lett. 1998; 102-103:277–282.
Article11. Wilhelm KP, Böttjer B, Siegers CP. Quantitative assessment of primary skin irritants in vitro in a cytotoxicity model: comparison with in vivo human irritation tests. Br J Dermatol. 2001; 145:709–715.
Article12. Engasser PG, Maibach HI. Cosmetic and dermatology: bleaching creams. J Am Acad Dermatol. 1981; 5:143–147.13. Yoshimura K, Momosawa A, Aiba E, Sato K, Matsumoto D, Mitoma Y, et al. Clinical trial of bleaching treatment with 10% all-trans retinol gel. Dermatol Surg. 2003; 29:155–160. discussion 160.
Article14. Nicholson M, Willis CM. The influence of patch test size and design on the distribution of erythema induced by sodium lauryl sulfate. Contact Dermatitis. 1999; 41:264–267.
Article15. Basketter D, Reynolds F, Rowson M, Talbot C, Whittle E. Visual assessment of human skin irritation: a sensitive and reproducible tool. Contact Dermatitis. 1997; 37:218–220.
Article16. Akhavan A, Bershad S. Topical acne drugs: review of clinical properties, systemic exposure, and safety. Am J Clin Dermatol. 2003; 4:473–492.17. De Groot AC, Frosch PJ. Patch test concentrations and vehicles for testing contact allergens. In : Frosch PJ, Menné T, Lepoittevin JP, editors. Contact dermatitis. 4th ed. Berlin/Heidelberg: Springer-Verlag Berlin Heidelberg;2006. p. 907–928.18. York M, Griffiths HA, Whittle E, Basketter DA. Evaluation of a human patch test for the identification and classification of skin irritation potential. Contact Dermatitis. 1996; 34:204–212.
Article19. Horita K, Tanoue C, Yasoshima M, Ohtani T, Matsunaga K. Study of the usefulness of patch testing and use test to predict the safety of commercial topical drugs. J Dermatol. 2014; 41:505–513.
Article20. Hannuksela A, Hannuksela M. Irritant effects of a detergent in wash, chamber and repeated open application tests. Contact Dermatitis. 1996; 34:134–137.
Article21. Gay R, Swiderek M, Nelson D, Ernesti A. The living skin equivalent as a model in vitro for ranking the toxic potential of dermal irritants. Toxicol In Vitro. 1992; 6:303–315.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Animal Experiments of Cutaneous Depigmentation by 4-Isoprophlcatechol and Hydroquinone
- IL-1 Receptor Antagonist Reduced Chemical-Induced Keratinocyte Apoptosis through Antagonism to IL-1α/IL-1β
- Allergic contact dermatitis to hydroquinone in bleaching cream
- Melasma
- Depigmentation Therapy with Monobenzyl Ether of Hydroquinone in a Patient with Vitiligo Universalis