J Rheum Dis.
2017 Oct;24(5):293-302. 10.4078/jrd.2017.24.5.293.
Cardiovascular and Gastrointestinal Effects of Etoricoxib in the Treatment of Osteoarthritis: A Systematic Review and Network Meta-analysis
- Affiliations
-
- 1Department of Rheumatology, Hanyang University Hospital for Rheumatic Diseases, Seoul, Korea. sungyk@hanyang.ac.kr
- 2College of Pharmacy, Chung-Ang University, Seoul, Korea.
- 3Division of Rheumatology, Department of Internal Medicine, Soon Chun Hyang University Bucheon Hospital, Bucheon, Korea.
- 4Department of Statistics, Kyungpook National University, Daegu, Korea.
- 5Department of Information Statistics, Andong National University, Andong, Korea.
- KMID: 2394345
- DOI: http://doi.org/10.4078/jrd.2017.24.5.293
Abstract
OBJECTIVE
To estimate the cardiovascular (CV) and gastrointestinal (GI) risks of etoricoxib in the treatment of osteoarthritis (OA) compared to a placebo and other non-steroidal anti-inflammatory drugs (NSAIDs).
METHODS
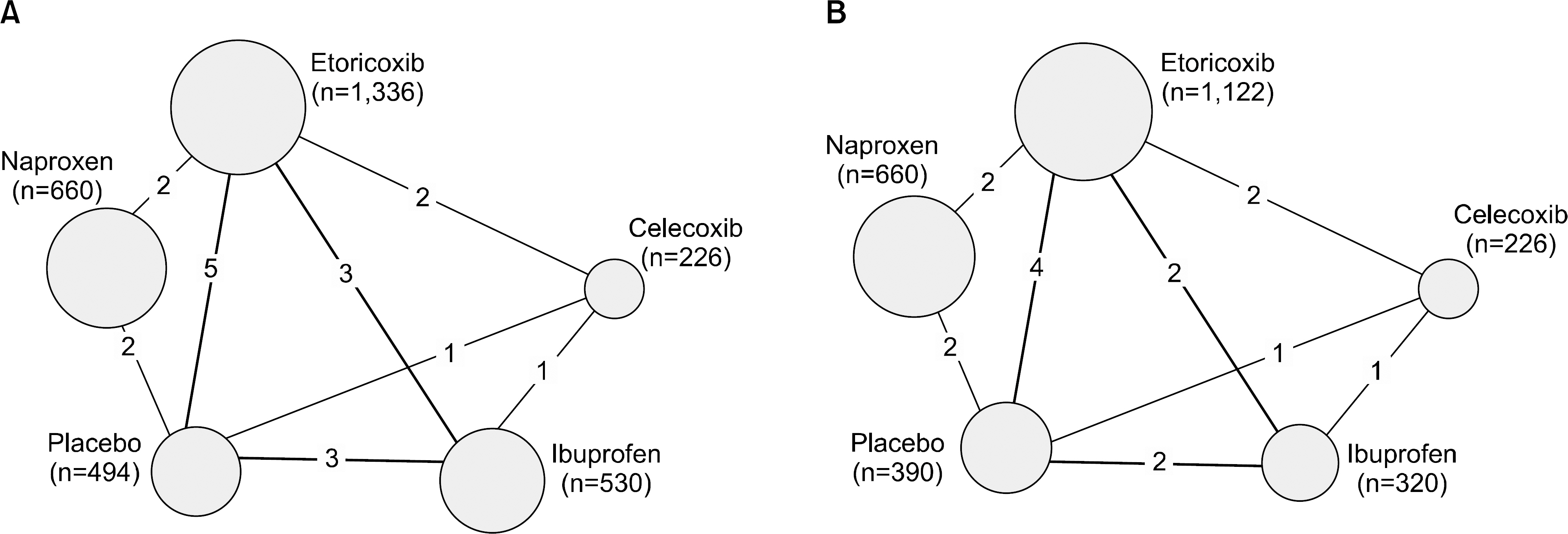
A systematic review of randomized, controlled trials (RCTs) of etoricoxib were performed. Bayesian network meta-analysis was used over a duration of 12 weeks. The incidence of CV and GI events for a duration ≥26 weeks were also tabulated and presented using descriptive statistics.
RESULTS
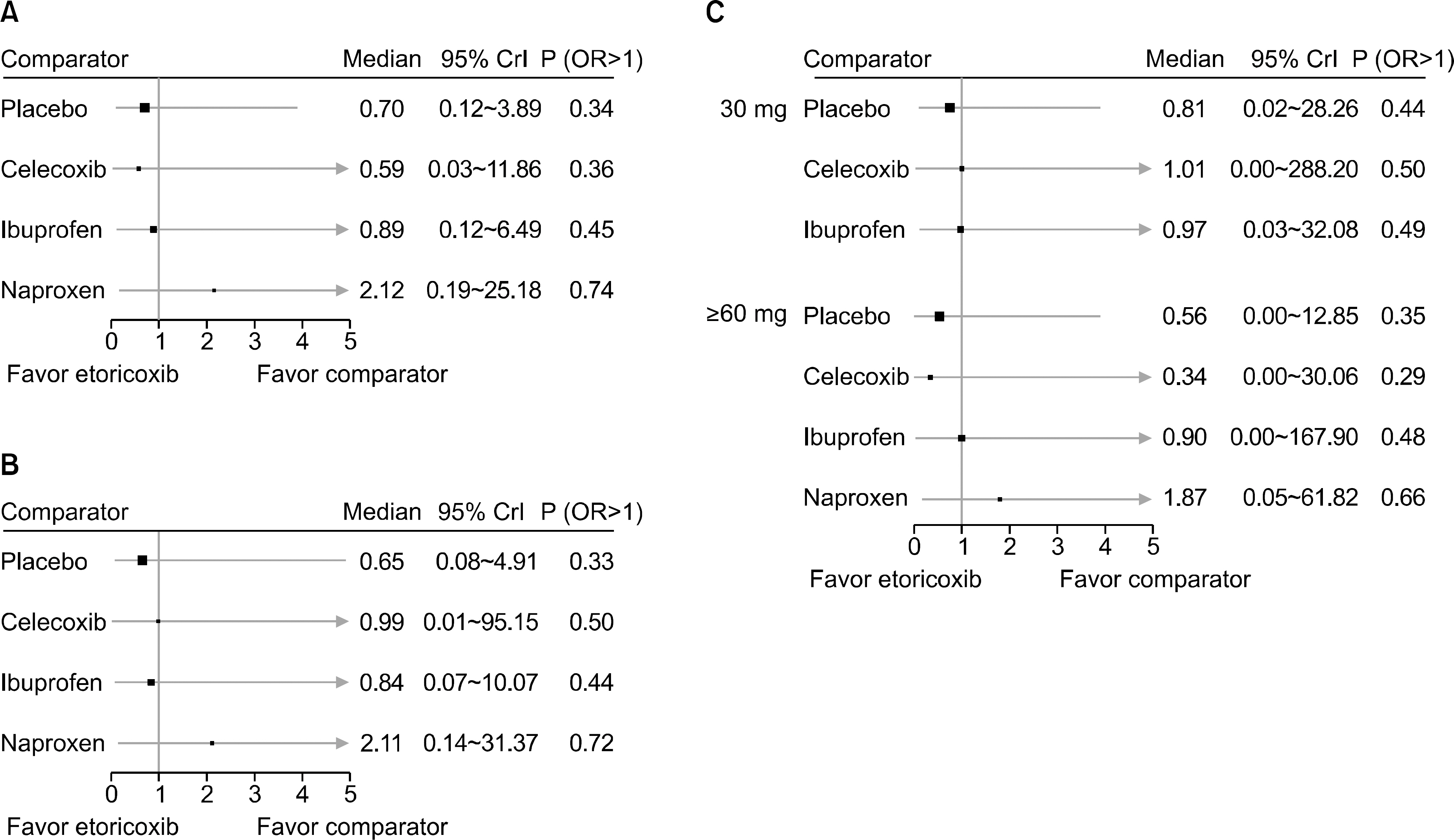
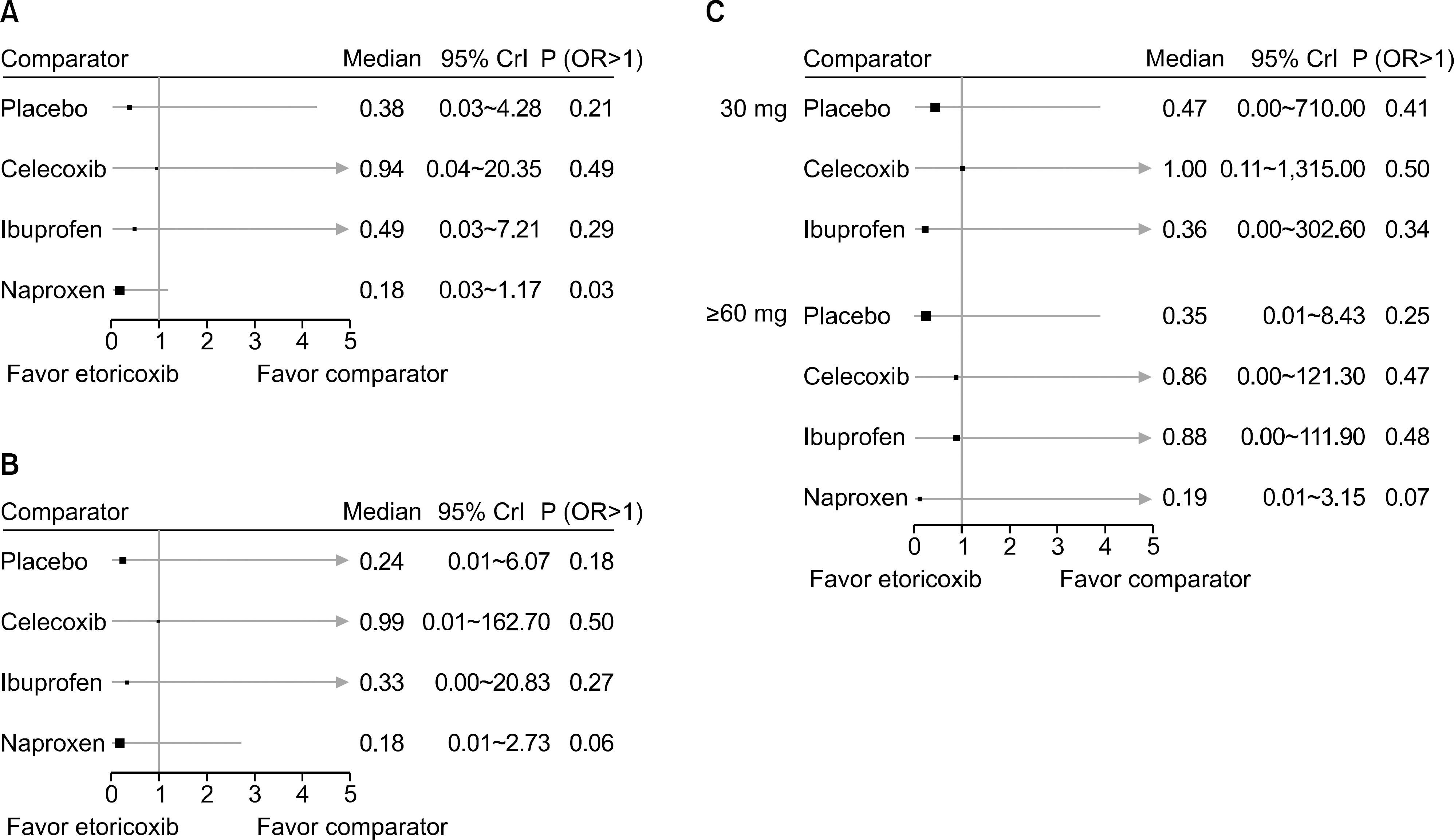
From this search, 10 studies were identified. Of these, 6 and 5 RCTs that measured the CV and GI events at 12 weeks were included in meta-analysis. They showed that etoricoxib did not increase the CV events compared to the placebo or NSAIDs during the 12 week period (odds ratio [OR]=0.59 compared to celecoxib, OR=0.89 with ibuprofen, OR=0.70 with placebo, and OR=2.16 with naproxen). The risk of GI events was comparable to that of most comparators, with the exception of naproxen, which had a significantly lower risk of GI events (OR=0.18) during the 12 week period. For a duration ≥26 weeks, the incidence of CV and GI events with etoricoxib increased with increasing duration.
CONCLUSION
Etoricoxib is an alternative short-term treatment option for OA, showing comparable CV and GI complications to other NSAIDs. Nevertheless, further studies will be needed to elucidate the long-term safety of etoricoxib in the treatment of OA.
MeSH Terms
Figure
Cited by 1 articles
-
Pharmacological treatment of osteoarthritis
Hyoungyoung Kim, Yoon-Kyoung Sung
J Korean Med Assoc. 2018;61(10):623-629. doi: 10.5124/jkma.2018.61.10.623.
Reference
-
1. Elders MJ. The increasing impact of arthritis on public health. J Rheumatol Suppl. 2000; 60:6–8.2. Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012; 64:465–74.
Article3. Jordan KM, Arden NK, Doherty M, Bannwarth B, Bijlsma JW, Dieppe P, et al. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis. 2003; 62:1145–55.
Article4. McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014; 22:363–88.
Article5. Jevsevar DS. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013; 21:571–6.
Article6. Wolfe MM, Lichtenstein DR, Singh G. Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. N Engl J Med. 1999; 340:1888–99.
Article7. Gabriel SE, Jaakkimainen L, Bombardier C. Risk for serious gastrointestinal complications related to use of non-steroidal anti-inflammatory drugs. A meta-analysis. Ann Intern Med. 1991; 115:787–96.8. Dannhardt G, Kiefer W. Cyclooxygenase inhibitors–current status and future prospects. Eur J Med Chem. 2001; 36:109–26.9. Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005; 352:1092–102.
Article10. Solomon DH, Avorn J, Stürmer T, Glynn RJ, Mogun H, Schneeweiss S. Cardiovascular outcomes in new users of coxibs and nonsteroidal antiinflammatory drugs: high-risk subgroups and time course of risk. Arthritis Rheum. 2006; 54:1378–89.
Article11. Abraham NS, El-Serag HB, Hartman C, Richardson P, Deswal A. Cyclooxygenase-2 selectivity of non-steroidal antiinflammatory drugs and the risk of myocardial infarction and cerebrovascular accident. Aliment Pharmacol Ther. 2007; 25:913–24.
Article12. Croom KF, Siddiqui MA. Etoricoxib: a review of its use in the symptomatic treatment of osteoarthritis, rheumatoid arthritis, ankylosing spondylitis and acute gouty arthritis. Drugs. 2009; 69:1513–32.13. van Walsem A, Pandhi S, Nixon RM, Guyot P, Karabis A, Moore RA. Relative benefit-risk comparing diclofenac to other traditional non-steroidal anti-inflammatory drugs and cyclooxygenase-2 inhibitors in patients with osteoarthritis or rheumatoid arthritis: a network meta-analysis. Arthritis Res Ther. 2015; 17:66.
Article14. Gunter BR, Butler KA, Wallace RL, Smith SM, Harirforoosh S. Non-steroidal anti-inflammatory drug-induced cardiovascular adverse events: a meta-analysis. J Clin Pharm Ther. 2017; 42:27–38.
Article15. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011; 343:d5928.
Article16. Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.1. 0 [updated March 2011] [Internet]. London: The Cochrane Collaboration;[cited 2017 Sep 12]. Available from:. http://handbook-5-1.cochrane.org/.17. Hoaglin DC, Hawkins N, Jansen JP, Scott DA, Itzler R, Cappelleri JC, et al. Conducting indirect-treatment-comparison and network-meta-analysis studies: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 2. Value Health. 2011; 14:429–37.
Article18. Sutton AJ, Abrams KR. Bayesian methods in meta-analysis and evidence synthesis. Stat Methods Med Res. 2001; 10:277–303.
Article19. Jansen JP, Fleurence R, Devine B, Itzler R, Barrett A, Hawkins N, et al. Interpreting indirect treatment comparisons and network meta-analysis for healthcare decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1. Value Health. 2011; 14:417–28.
Article20. Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010; 29:932–44.
Article21. Baraf HS, Fuentealba C, Greenwald M, Brzezicki J, O'Brien K, Soffer B, et al. Gastrointestinal side effects of etoricoxib in patients with osteoarthritis: results of the Etoricoxib versus Diclofenac Sodium Gastrointestinal Tolerability and Effectiveness (EDGE) trial. J Rheumatol. 2007; 34:408–20.22. Bingham CO 3rd, Sebba AI, Rubin BR, Ruoff GE, Kremer J, Bird S, et al. Efficacy and safety of etoricoxib 30 mg and celecoxib 200 mg in the treatment of osteoarthritis in two iden-tically designed, randomized, placebo-controlled, non-inferiority studies. Rheumatology (Oxford). 2007; 46:496–507.
Article23. Cannon CP, Chen C, Curtis SP, Viscusi J, Ahmed T, Dibattiste PM. A comparison of cardiovascular biomarkers in patients treated for three months with etoricoxib, celecoxib, ibuprofen, and placebo. Arch Drug Inf. 2008; 1:4–13.
Article24. Curtis SP, Bockow B, Fisher C, Olaleye J, Compton A, Ko AT, et al. Etoricoxib in the treatment of osteoarthritis over 52-weeks: a double-blind, active-comparator controlled trial [NCT00242489]. BMC Musculoskelet Disord. 2005; 6:58.
Article25. Gottesdiener K, Schnitzer T, Fisher C, Bockow B, Marken-son J, Ko A, et al. Results of a randomized, doseranging trial of etoricoxib in patients with osteoarthritis. Rheumatology (Oxford). 2002; 41:1052–61.
Article26. Leung AT, Malmstrom K, Gallacher AE, Sarembock B, Poor G, Beaulieu A, et al. Efficacy and tolerability profile of etoricoxib in patients with osteoarthritis: A randomized, dou-ble-blind, placebo and active-comparator controlled 12-week efficacy trial. Curr Med Res Opin. 2002; 18:49–58.
Article27. Puopolo A, Boice JA, Fidelholtz JL, Littlejohn TW, Miranda P, Berrocal A, et al. A randomized placebo-controlled trial comparing the efficacy of etoricoxib 30 mg and ibuprofen 2400 mg for the treatment of patients with osteoarthritis. Osteoarthritis Cartilage. 2007; 15:1348–56.28. Reginster JY, Malmstrom K, Mehta A, Bergman G, Ko AT, Curtis SP, et al. Evaluation of the efficacy and safety of etoricoxib compared with naproxen in two, 138-week randomised studies of patients with osteoarthritis. Ann Rheum Dis. 2007; 66:945–51.
Article29. Wiesenhutter CW, Boice JA, Ko A, Sheldon EA, Murphy FT, Wittmer BA, et al. Evaluation of the comparative efficacy of etoricoxib and ibuprofen for treatment of patients with osteoarthritis: A randomized, double-blind, placebo-controlled trial. Mayo Clin Proc. 2005; 80:470–9.
Article30. Yoo MC, Yoo WH, Kang SB, Park YW, Kim SS, Moon KH, et al. Etoricoxib in the treatment of Korean patients with osteoarthritis in a double-blind, randomized controlled trial. Curr Med Res Opin. 2014; 30:2399–408.
Article31. García Rodríguez LA, Barreales Tolosa L. Risk of upper gastrointestinal complications among users of traditional NSAIDs and COXIBs in the general population. Gastroenterology. 2007; 132:498–506.
Article32. Nussmeier NA, Whelton AA, Brown MT, Langford RM, Hoeft A, Parlow JL, et al. Complications of the COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery. N Engl J Med. 2005; 352:1081–91.
Article33. Warner TD, Mitchell JA. Cyclooxygenases: new forms, new inhibitors, and lessons from the clinic. FASEB J. 2004; 18:790–804.
Article34. Dallob A, Hawkey CJ, Greenberg H, Wight N, De Schepper P, Waldman S, et al. Characterization of etoricoxib, a novel, selective COX-2 inhibitor. J Clin Pharmacol. 2003; 43:573–85.
Article35. Lin HY, Cheng TT, Wang JH, Lee CS, Chen MH, Lei V, et al. Etoricoxib improves pain, function and quality of life: results of a real-world effectiveness trial. Int J Rheum Dis. 2010; 13:144–50.
Article36. Turajane T, Wongbunnak R, Patcharatrakul T, Ratansuma-wong K, Poigampetch Y, Songpatanasilp T. Gastrointestinal and cardiovascular risk of nonselective NSAIDs and COX-2 inhibitors in elderly patients with knee osteoarthritis. J Med Assoc Thai. 2009; 92(Suppl 6):S19–26.37. Sutton AJ, Cooper NJ, Lambert PC, Jones DR, Abrams KR, Sweeting MJ. Meta-analysis of rare and adverse event data. Expert Rev Pharmacoecon Outcomes Res. 2002; 2:367–79.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Concepts and emerging issues of network meta-analysis
- Trends of Meta-analysis in Upper Gastrointestinal Diseases
- Effect of Stretching Exercises on Patients with Knee Osteoarthritis: A Systematic Review and Meta-Analysis
- Meta-epidemiology
- Comparative Efficacy of Clinical Interventions for Sacroiliac Joint Pain: Systematic Review and Network Meta-analysis With Preliminary Design of Treatment Algorithm