J Korean Soc Radiol.
2017 Nov;77(5):317-326. 10.3348/jksr.2017.77.5.317.
A Study of Feasibility of Brain Imaging in Medium- and Small-Sized Animals: Using a Clinical 3T MR System with Three Surface Coils
- Affiliations
-
- 1Department of Radiology, Pusan National University Hospital, Pusan National University College of Medicine, Busan, Korea. hakjink@pusan.ac.kr
- 2Department of Preventive Medicine, Pusan National University College of Medicine, Busan, Korea.
- 3MR Application Specialist, Siemens Healthcare Korea, Seoul, Korea.
- KMID: 2394046
- DOI: http://doi.org/10.3348/jksr.2017.77.5.317
Abstract
- PURPOSE
To evaluate which brain MR images obtained with a clinical 3T MR system using surface coils less than 15.4 cm in diameter are best in rabbit and rat models, and to assess the feasibility of the clinical 3T MR machine in the study of morphologic brain in a preclinical study using medium- and small-sized animal models.
MATERIALS AND METHODS
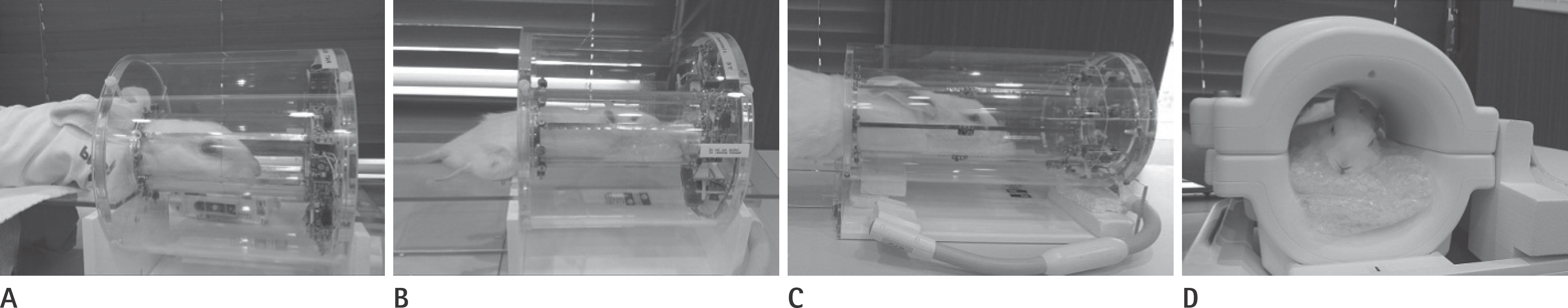
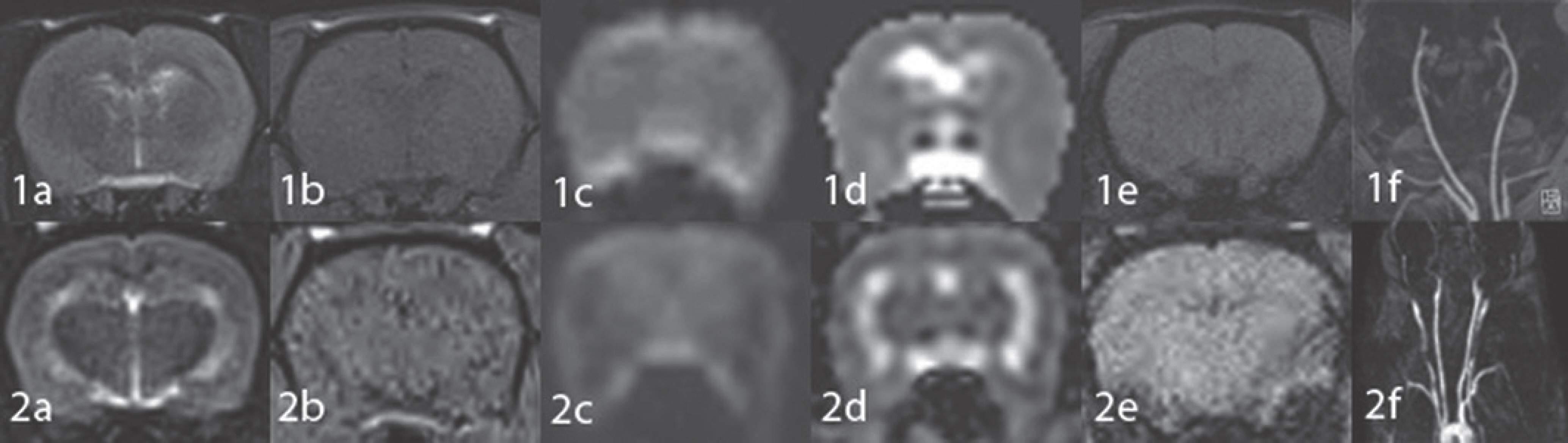
Brain T2-weighted image (T2WI), T1-weighted image (T1WI), diffusion-weighted image (DWI), and susceptibility-weighted image (SWI) were obtained, and MR angiography was performed with a clinical 3T MR system using a rat, a cat, and a knee coil (5, 12, and 15.4 cm in diameter, respectively) in normal rabbits (n = 3) and using a rat and a cat coil in normal rats (n = 3). MR images were assessed qualitatively by consensus of two neuroradiologists and quantitatively using signal-to-noise ratio (SNR) and statistical analysis (using analysis of variance or t-test) in terms of which images obtained with different coils were the best. Brain T2WI, DWI, SWI, and Gd-T1WI MR images were obtained 2 hours after embolization with triolein emulsion infused into the carotid artery in rabbits (n = 3) and rats (n = 3) using the coil which showed highest SNR in the above study, and the images were assessed in terms of abnormal findings and image quality.
RESULTS
Brain MR images obtained with the rat coil revealed better image quality and higher SNR compared with those obtained with other coils, and they showed statistical significance (p < 0.05) in rabbits. In rats, brain MR images obtained with the rat coil were better than those obtained with the cat coil in qualitative analysis; however, they revealed no statistical significance except for DWI in quantitative analysis. MR images obtained after triolein emulsion showed T2 hyperintensity and lesional contrast enhancement on Gd-T1WI without evidence of infarction or hemorrhage.
CONCLUSION
The clinical 3T MR system using surface coils for animals enabled us to obtain good quality brain images in medium- and small-sized animal models in the present study. Brain MR images seem to be feasible for the morphologic evaluation in animal models.
MeSH Terms
Figure
Reference
-
1.Liang CC., Liu HL., Chang SD., Chen SH., Lee TH. The protective effect of human umbilical cord blood CD34+ cells and es-tradiol against focal cerebral ischemia in female ovariecto-mized rat: cerebral MR imaging and immunohistochemical study. PLoS One. 2016. 11:e0147133.
Article2.Bearer EL., Zhang X., Janvelyan D., Boulat B., Jacobs RE. Reward circuitry is perturbed in the absence of the serotonin trans-porter. Neuroimage. 2009. 46:1091–1104.
Article3.Kim HJ., Lee CH., Kim HG., Lee SD., Son SM., Kim YW, et al. Re-versible MR changes in the cat brain after cerebral fat em-bolism induced by triolein emulsion. AJNR Am J Neuroradiol. 2004. 25:958–963.4.Kim HJ., Lee CH., Lee SH., Cho BM., Kim HK., Park BR, et al. Early development of vasogenic edema in experimental cerebral fat embolism in cats: correlation with MRI and electron microscopic findings. Invest Radiol. 2001. 36:460–469.5.Kim HJ., Lee CH., Lee SH., Moon TY. Magnetic resonance im-aging and histologic findings of experimental cerebral fat embolism. Invest Radiol. 2003. 38:625–634.
Article6.Kim HJ., Lee JH., Lee CH., Lee SH., Moon TY., Cho BM, et al. Ex-perimental cerebral fat embolism: embolic effects of triole-in and oleic acid depicted by MR imaging and electron mi-croscopy. AJNR Am J Neuroradiol. 2002. 23:1516–1523.7.Kim HJ., Pyeun YS., Kim YW., Cho BM., Lee TH., Moon TY, et al. A model for research on the blood-brain barrier disruption induced by unsaturated fatty acid emulsion. Invest Radiol. 2005. 40:270–276.
Article8.Kim HJ., Kim YW., Choi SH., Cho BM., Bandu R., Ahn HS, et al. Triolein emulsion infusion into the carotid artery increases brain permeability to anticancer agents. Neurosurgery. 2016. 78:726–733.
Article9.Lee IS., Lee JE., Kim HJ., Song JW., Choi SH. Immediate break-down of blood retinal barrier by infusion of triolein emulsion observed by fluorescein angiography. Curr Eye Res. 2011. 36:358–363.
Article10.Lee JE., Jea SY., Oum BS., Kim HJ., Ohn YH. Effect of fat embo-lism with triolein emulsion on blood-retinal barrier. Oph-thalmic Res. 2009. 41:14–20.
Article11.Lee JY., Eun CK., Kim YW., Kim HJ., Jung YJ., Jae SY, et al. The steroid effect on the blood-ocular barrier change induced by triolein emulsion as seen on contrast-enhanced MR im-ages. Korean J Radiol. 2008. 9:205–211.
Article12.Kim YW., Kim HJ., Cho BM., Moon TY., Eun CK. The study of ce-rebral hemodynamics in the hyperacute stage of fat embo-lism induced by triolein emulsion. AJNR Am J Neuroradiol. 2006. 27:398–401.13.Kim YW., Park YM., Yoon S., Kim HJ., Park DY., Cho BM, et al. Effect of intra-arterial infusion with triolein emulsion on rabbit liver. World J Gastroenterol. 2014. 20:14442–14449.
Article14.Haenold R., Herrmann KH., Schmidt S., Reichenbach JR., Schmidt KF., Löwel S, et al. Magnetic resonance imaging of the mouse visual pathway for in vivo studies of degeneration and re-generation in the CNS. Neuroimage. 2012. 59:363–376.
Article15.Chang NK., Jeong YY., Park JS., Jeong HS., Jang S., Jang MJ, et al. Tracking of neural stem cells in rats with intracerebral hemorrhage by the use of 3T MRI. Korean J Radiol. 2008. 9:196–204.
Article16.Wu B., Wang C., Pang Y., Zhang X. Comparison of SNR calcu-lation methods for in vivo imaging. Available at:. http://cds.ismrm.org/protected/10MProceedings/files/3153_1012.pdf. Accessed Mar 4, 2017.17.Pfeuffer J., Merkle H., Beyerlein M., Steudel T., Logothetis NK. Anatomical and functional MR imaging in the macaque mon-key using a vertical large-bore 7 Tesla setup. Magn Reson Imaging. 2004. 22:1343–1359.
Article18.Mezer A., Yeatman JD., Stikov N., Kay KN., Cho NJ., Dougherty RF, et al. Quantifying the local tissue volume and composition in individual brains with magnetic resonance imaging. Nat Med. 2013. 19:1667–1672.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- High field strength magnetic resonance imaging of abdominal diseases
- Comparison of Surface and Saddle Endoanal Coil to Evaluate anal Sphincter in Infants and Young Children: Experimental Study Using Phantom and Cats
- Moderating Effects of Work-family Conflict between Job . Organizational . Career Characteristics and Turnover Intention among Nurses in Small and Medium-sized Hospitals
- Imaging Studies in Mouse Brain Using Clinical 3T MRI Scanner
- Establishing a Clinical Ladder System for Nurses in a Small and Medium-sized Hospital