Diabetes Metab J.
2017 Oct;41(5):327-336. 10.4093/dmj.2017.41.5.327.
Skeletal Muscle Thermogenesis and Its Role in Whole Body Energy Metabolism
- Affiliations
-
- 1Sanford Burnham Prebys Medical Discovery Institute at Lake Nona, Orlando, FL, USA. mperiasamy@SBPdiscovery.org
- KMID: 2392475
- DOI: http://doi.org/10.4093/dmj.2017.41.5.327
Abstract
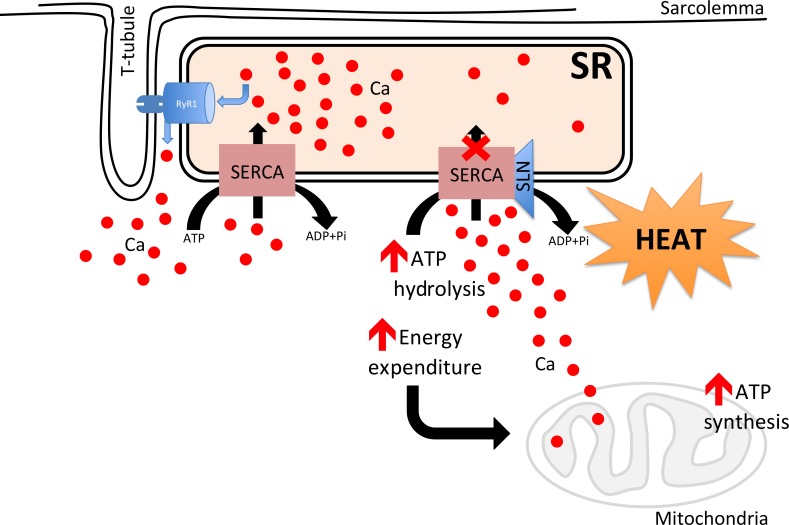
- Obesity and diabetes has become a major epidemic across the globe. Controlling obesity has been a challenge since this would require either increased physical activity or reduced caloric intake; both are difficult to enforce. There has been renewed interest in exploiting pathways such as uncoupling protein 1 (UCP1)-mediated uncoupling in brown adipose tissue (BAT) and white adipose tissue to increase energy expenditure to control weight gain. However, relying on UCP1-based thermogenesis alone may not be sufficient to control obesity in humans. On the other hand, skeletal muscle is the largest organ and a major contributor to basal metabolic rate and increasing energy expenditure in muscle through nonshivering thermogenic mechanisms, which can substantially affect whole body metabolism and weight gain. In this review we will describe the role of Sarcolipin-mediated uncoupling of Sarcoplasmic Reticulum Calcium ATPase (SERCA) as a potential mechanism for increased energy expenditure both during cold and diet-induced thermogenesis.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Umbilical Cord-Mesenchymal Stem Cell-Conditioned Medium Improves Insulin Resistance in C2C12 Cell
Kyung-Soo Kim, Yeon Kyung Choi, Mi Jin Kim, Jung Wook Hwang, Kyunghoon Min, Sang Youn Jung, Soo-Kyung Kim, Yong-Soo Choi, Yong-Wook Cho
Diabetes Metab J. 2021;45(2):260-269. doi: 10.4093/dmj.2019.0191.Exercise, Mitohormesis, and Mitochondrial ORF of the 12S rRNA Type-C (MOTS-c)
Tae Kwan Yoon, Chan Hee Lee, Obin Kwon, Min-Seon Kim
Diabetes Metab J. 2022;46(3):402-413. doi: 10.4093/dmj.2022.0092.
Reference
-
1. Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001; 104:531–543. PMID: 11239410.
Article2. Lepor NE, Fouchia DD, McCullough PA. New vistas for the treatment of obesity: turning the tide against the leading cause of morbidity and cardiovascular mortality in the developed world. Rev Cardiovasc Med. 2013; 14:20–39. PMID: 23651984.3. Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon-Moran D, Kit BK, Flegal KM. Trends in obesity prevalence among children and adolescents in the United States, 1988-1994 through 2013-2014. JAMA. 2016; 315:2292–2299. PMID: 27272581.
Article4. Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011-2014. NCHS Data Brief. 2015; (219):1–8.5. Padwal R, Li SK, Lau DC. Long-term pharmacotherapy for overweight and obesity: a systematic review and meta-analysis of randomized controlled trials. Int J Obes Relat Metab Disord. 2003; 27:1437–1446. PMID: 12975638.
Article6. Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol (1985). 2000; 89:81–88. PMID: 10904038.
Article7. Zurlo F, Nemeth PM, Choksi RM, Sesodia S, Ravussin E. Whole-body energy metabolism and skeletal muscle biochemical characteristics. Metabolism. 1994; 43:481–486. PMID: 8159108.
Article8. Zurlo F, Larson K, Bogardus C, Ravussin E. Skeletal muscle metabolism is a major determinant of resting energy expenditure. J Clin Invest. 1990; 86:1423–1427. PMID: 2243122.
Article9. Ferrannini E, Simonson DC, Katz LD, Reichard G Jr, Bevilacqua S, Barrett EJ, Olsson M, DeFronzo RA. The disposal of an oral glucose load in patients with non-insulin-dependent diabetes. Metabolism. 1988; 37:79–85. PMID: 3275860.
Article10. Kelley DE. Skeletal muscle fat oxidation: timing and flexibility are everything. J Clin Invest. 2005; 115:1699–1702. PMID: 16007246.
Article11. Turner N, Cooney GJ, Kraegen EW, Bruce CR. Fatty acid metabolism, energy expenditure and insulin resistance in muscle. J Endocrinol. 2014; 220:T61–T79. PMID: 24323910.
Article12. Thiebaud D, Jacot E, DeFronzo RA, Maeder E, Jequier E, Felber JP. The effect of graded doses of insulin on total glucose uptake, glucose oxidation, and glucose storage in man. Diabetes. 1982; 31:957–963. PMID: 6757014.
Article13. Pant M, Bal NC, Periasamy M. Sarcolipin: a key thermogenic and metabolic regulator in skeletal muscle. Trends Endocrinol Metab. 2016; 27:881–892. PMID: 27637585.
Article14. Periasamy M, Maurya SK, Sahoo SK, Singh S, Sahoo SK, Reis FCG, Bal NC. Role of SERCA pump in muscle thermogenesis and metabolism. Compr Physiol. 2017; 7:879–890. PMID: 28640447.
Article15. Lowell BB, Bachman ES. Beta-adrenergic receptors, diet-induced thermogenesis, and obesity. J Biol Chem. 2003; 278:29385–29388. PMID: 12788929.16. Maurya SK, Periasamy M. Sarcolipin is a novel regulator of muscle metabolism and obesity. Pharmacol Res. 2015; 102:270–275. PMID: 26521759.
Article17. Sahoo SK, Shaikh SA, Sopariwala DH, Bal NC, Periasamy M. Sarcolipin protein interaction with sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA) is distinct from phospholamban protein, and only sarcolipin can promote uncoupling of the SERCA pump. J Biol Chem. 2013; 288:6881–6889. PMID: 23341466.18. Sahoo SK, Shaikh SA, Sopariwala DH, Bal NC, Bruhn DS, Kopec W, Khandelia H, Periasamy M. The N terminus of sarcolipin plays an important role in uncoupling sarco-endoplasmic reticulum Ca2+-ATPase (SERCA) ATP hydrolysis from Ca2+ transport. J Biol Chem. 2015; 290:14057–14067. PMID: 25882845.
Article19. Shaikh SA, Sahoo SK, Periasamy M. Phospholamban and sarcolipin: are they functionally redundant or distinct regulators of the sarco(endo)plasmic reticulum calcium ATPase? J Mol Cell Cardiol. 2016; 91:81–91. PMID: 26743715.
Article20. Bal NC, Maurya SK, Sopariwala DH, Sahoo SK, Gupta SC, Shaikh SA, Pant M, Rowland LA, Bombardier E, Goonasekera SA, Tupling AR, Molkentin JD, Periasamy M. Sarcolipin is a newly identified regulator of muscle-based thermogenesis in mammals. Nat Med. 2012; 18:1575–1579. PMID: 22961106.
Article21. Babu GJ, Bhupathy P, Timofeyev V, Petrashevskaya NN, Reiser PJ, Chiamvimonvat N, Periasamy M. Ablation of sarcolipin enhances sarcoplasmic reticulum calcium transport and atrial contractility. Proc Natl Acad Sci U S A. 2007; 104:17867–17872. PMID: 17971438.
Article23. Rowland LA, Bal NC, Periasamy M. The role of skeletal-muscle-based thermogenic mechanisms in vertebrate endothermy. Biol Rev Camb Philos Soc. 2015; 90:1279–1297. PMID: 25424279.
Article24. Runcie RM, Dewar H, Hawn DR, Frank LR, Dickson KA. Evidence for cranial endothermy in the opah (Lampris guttatus). J Exp Biol. 2009; 212(Pt 4):461–470. PMID: 19181893.
Article25. Rosenberg H, Pollock N, Schiemann A, Bulger T, Stowell K. Malignant hyperthermia: a review. Orphanet J Rare Dis. 2015; 10:93. PMID: 26238698.
Article26. MacLennan DH. The genetic basis of malignant hyperthermia. Trends Pharmacol Sci. 1992; 13:330–334. PMID: 1329295.
Article27. MacLennan DH, Otsu K, Fujii J, Zorzato F, Phillips MS, O'Brien PJ, Archibald AL, Britt BA, Gillard EF, Worton RG. The role of the skeletal muscle ryanodine receptor gene in malignant hyperthermia. Symp Soc Exp Biol. 1992; 46:189–201. PMID: 1341035.28. MacLennan DH, Phillips MS. Malignant hyperthermia. Science. 1992; 256:789–794. PMID: 1589759.
Article29. Sharma A, Karnik H, Kukreja S, Jagger K. Malignant hyperthermia: dantrolene sodium. A must have. Indian J Anaesth. 2012; 56:212–213. PMID: 22701231.30. Das AM, Steuerwald U, Illsinger S. Inborn errors of energy metabolism associated with myopathies. J Biomed Biotechnol. 2010; 2010:340849. PMID: 20589068.
Article31. Cannon B, Houstek J, Nedergaard J. Brown adipose tissue. More than an effector of thermogenesis? Ann N Y Acad Sci. 1998; 856:171–187. PMID: 9917877.32. Cannon B, Jacobsson A, Rehnmark S, Nedergaard J. Signal transduction in brown adipose tissue recruitment: noradrenaline and beyond. Int J Obes Relat Metab Disord. 1996; 20(Suppl 3):S36–S42. PMID: 8680475.33. Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004; 84:277–359. PMID: 14715917.
Article34. Kozak LP, Harper ME. Mitochondrial uncoupling proteins in energy expenditure. Annu Rev Nutr. 2000; 20:339–363. PMID: 10940338.
Article35. Meyer CW, Willershauser M, Jastroch M, Rourke BC, Fromme T, Oelkrug R, Heldmaier G, Klingenspor M. Adaptive thermogenesis and thermal conductance in wild-type and UCP1-KO mice. Am J Physiol Regul Integr Comp Physiol. 2010; 299:R1396–R1406. PMID: 20826705.
Article36. Kozak LP, Anunciado-Koza R. UCP1: its involvement and utility in obesity. Int J Obes (Lond). 2008; 32(Suppl 7):S32–S38. PMID: 19136989.
Article37. Kozak LP, Koza RA. Mitochondria uncoupling proteins and obesity: molecular and genetic aspects of UCP1. Int J Obes Relat Metab Disord. 1999; 23(Suppl 6):S33–S37. PMID: 10454119.38. Duchamp C, Barre H. Skeletal muscle as the major site of nonshivering thermogenesis in cold-acclimated ducklings. Am J Physiol. 1993; 265(5 Pt 2):R1076–R1083. PMID: 8238608.
Article39. Duchamp C, Cohen-Adad F, Rouanet JL, Barre H. Histochemical arguments for muscular non-shivering thermogenesis in muscovy ducklings. J Physiol. 1992; 457:27–45. PMID: 1297835.
Article40. Dumonteil E, Barre H, Meissner G. Sarcoplasmic reticulum Ca(2+)-ATPase and ryanodine receptor in cold-acclimated ducklings and thermogenesis. Am J Physiol. 1993; 265(2 Pt 1):C507–C513. PMID: 8018125.
Article41. Arruda AP, Nigro M, Oliveira GM, de Meis L. Thermogenic activity of Ca2+-ATPase from skeletal muscle heavy sarcoplasmic reticulum: the role of ryanodine Ca2+ channel. Biochim Biophys Acta. 2007; 1768:1498–1505. PMID: 17466935.
Article42. Arruda AP, Ketzer LA, Nigro M, Galina A, Carvalho DP, de Meis L. Cold tolerance in hypothyroid rabbits: role of skeletal muscle mitochondria and sarcoplasmic reticulum Ca2+ ATPase isoform 1 heat production. Endocrinology. 2008; 149:6262–6271. PMID: 18703625.
Article43. de Meis L, Arruda AP, Carvalho DP. Role of sarco/endoplasmic reticulum Ca(2+)-ATPase in thermogenesis. Biosci Rep. 2005; 25:181–190. PMID: 16283552.
Article44. Periasamy M, Kalyanasundaram A. SERCA pump isoforms: their role in calcium transport and disease. Muscle Nerve. 2007; 35:430–442. PMID: 17286271.
Article45. Periasamy M, Huke S. SERCA pump level is a critical determinant of Ca(2+)homeostasis and cardiac contractility. J Mol Cell Cardiol. 2001; 33:1053–1063. PMID: 11444913.
Article46. Babu GJ, Bhupathy P, Carnes CA, Billman GE, Periasamy M. Differential expression of sarcolipin protein during muscle development and cardiac pathophysiology. J Mol Cell Cardiol. 2007; 43:215–222. PMID: 17561107.
Article47. Mall S, Broadbridge R, Harrison SL, Gore MG, Lee AG, East JM. The presence of sarcolipin results in increased heat production by Ca(2+)-ATPase. J Biol Chem. 2006; 281:36597–36602. PMID: 17018526.
Article48. Smith WS, Broadbridge R, East JM, Lee AG. Sarcolipin uncouples hydrolysis of ATP from accumulation of Ca2+ by the Ca2+-ATPase of skeletal-muscle sarcoplasmic reticulum. Biochem J. 2002; 361(Pt 2):277–286. PMID: 11772399.
Article49. Sopariwala DH, Pant M, Shaikh SA, Goonasekera SA, Molkentin JD, Weisleder N, Ma J, Pan Z, Periasamy M. Sarcolipin overexpression improves muscle energetics and reduces fatigue. J Appl Physiol (1985). 2015; 118:1050–1058. PMID: 25701006.
Article50. Ukropec J, Anunciado RP, Ravussin Y, Hulver MW, Kozak LP. UCP1-independent thermogenesis in white adipose tissue of cold-acclimated Ucp1-/- mice. J Biol Chem. 2006; 281:31894–31908. PMID: 16914547.
Article51. Bal NC, Maurya SK, Singh S, Wehrens XH, Periasamy M. Increased reliance on muscle-based thermogenesis upon acute minimization of brown adipose tissue function. J Biol Chem. 2016; 291:17247–17257. PMID: 27298322.
Article52. Rowland LA, Bal NC, Kozak LP, Periasamy M. Uncoupling protein 1 and sarcolipin are required to maintain optimal thermogenesis, and loss of both systems compromises survival of mice under cold stress. J Biol Chem. 2015; 290:12282–12289. PMID: 25825499.
Article53. Bal NC, Singh S, Reis FCG, Maurya SK, Pani S, Rowland LA, Periasamy M. Both brown adipose tissue and skeletal muscle thermogenesis processes are activated during mild to severe cold adaptation in mice. J Biol Chem. 2017; 292:16616–16625. PMID: 28794154.
Article54. Rothwell NJ, Stock MJ. Diet-induced thermogenesis. Adv Nutr Res. 1983; 5:201–220. PMID: 6342342.
Article55. Stock MJ. The role of brown adipose tissue in diet-induced thermogenesis. Proc Nutr Soc. 1989; 48:189–196. PMID: 2678114.
Article56. Kozak LP. Brown fat and the myth of diet-induced thermogenesis. Cell Metab. 2010; 11:263–267. PMID: 20374958.
Article57. Kozak LP, Koza RA, Anunciado-Koza R. Brown fat thermogenesis and body weight regulation in mice: relevance to humans. Int J Obes (Lond). 2010; 34(Suppl 1):S23–S27. PMID: 20935661.
Article58. Rothwell NJ, Stock MJ. Sympathetic and adrenocorticoid influences on diet-induced thermogenesis and brown fat activity in the rat. Comp Biochem Physiol A Comp Physiol. 1984; 79:575–579. PMID: 6150795.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Increaseing Physical Activity in Daily Life
- Connecting Myokines and Metabolism
- Mechanism of Elevated Oxygen Consumption by Intralipid and Heparin Injection: Increased Skeletal Muscle Fat Oxidation and UCP3 Expression
- Brown Adipose Tissue as a Regulator of Energy Expenditure and Body Fat in Humans
- Brown Fat as a Regulator of Systemic Metabolism beyond Thermogenesis