J Clin Neurol.
2017 Oct;13(4):359-365. 10.3988/jcn.2017.13.4.359.
Ultrastructural Changes in Skeletal Muscle of Infants with Mitochondrial Respiratory Chain Complex I Defects
- Affiliations
-
- 1Department of Biomedical Laboratory Science, College of Health Sciences, Eulji University, Seongnam, Korea.
- 2School of Life Sciences and Biotechnology, Korea University, Seoul, Korea.
- 3Department of Pathology, Yonsei University College of Medicine, Seoul, Korea.
- 4Epilepsy Research Institute, Yonsei University College of Medicine, Seoul, Korea.
- 5Department of Pediatrics, Yonsei University College of Medicine, Seoul, Korea. ymleemd@yuhs.ac
- KMID: 2392131
- DOI: http://doi.org/10.3988/jcn.2017.13.4.359
Abstract
- BACKGROUND AND PURPOSE
The pathogenesis of mitochondrial disease (MD) involves the disruption of cellular energy metabolism, which results from defects in the mitochondrial respiratory chain complex (MRC). We investigated whether infants with MRC I defects showed ultrastructural changes in skeletal muscle.
METHODS
Twelve infants were enrolled in this study. They were initially evaluated for unexplained neurodegenerative symptoms, myopathies, or other progressive multiorgan involvement, and underwent muscle biopsies when MD was suspected. Muscle tissue samples were subjected to biochemical enzyme assays and observation by transmission electron microscopy. We compared and analyzed the ultrastructure of skeletal muscle tissues obtained from patients with and without MRC I defects.
RESULTS
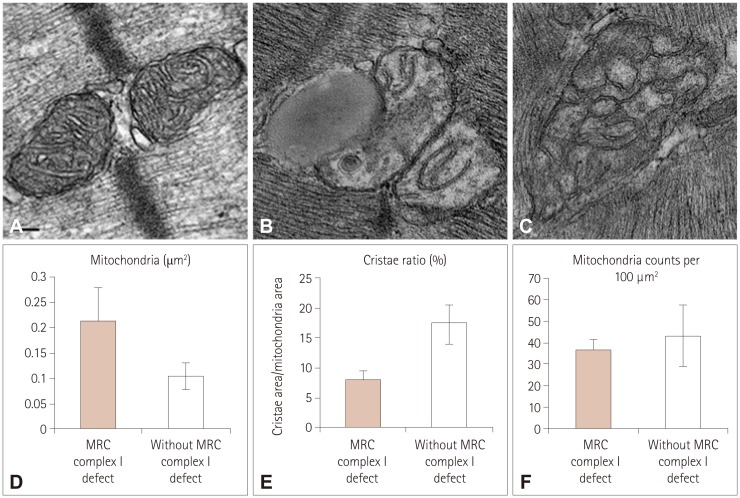
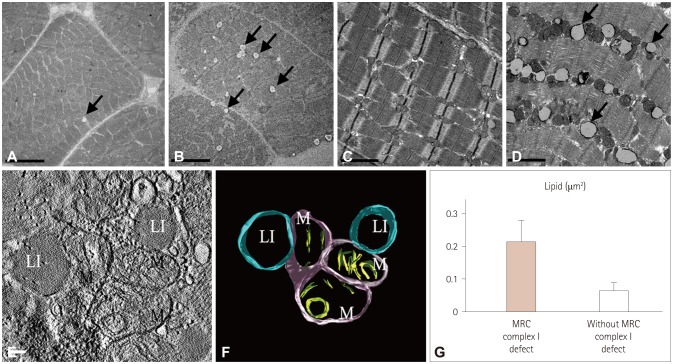
Biochemical enzyme assays confirmed the presence of MRC I defects in 7 of the 12 patients. Larger mitochondria, lipid droplets, and fused structures between the outer mitochondrial membrane and lipid droplets were observed in the skeletal muscles of patients with MRC I defects.
CONCLUSIONS
Mitochondrial functional defects in MRC I disrupt certain activities related to adenosine triphosphate synthesis that produce changes in the skeletal muscle. The ultrastructural changes observed in the infants in this study might serve as unique markers for the detection of MD.
Keyword
MeSH Terms
Figure
Reference
-
1. McFarland R, Taylor RW, Turnbull DM. The neurology of mitochondrial DNA disease. Lancet Neurol. 2002; 1:343–351. PMID: 12849395.
Article2. DiMauro S, Schon EA. Mitochondrial respiratory-chain diseases. N Engl J Med. 2003; 348:2656–2668. PMID: 12826641.
Article4. Loeffen JL, Smeitink JA, Trijbels JM, Janssen AJ, Triepels RH, Sengers RC, et al. Isolated complex I deficiency in children: clinical, biochemical and genetic aspects. Hum Mutat. 2000; 15:123–134. PMID: 10649489.
Article5. Miles L, Wong BL, Dinopoulos A, Morehart PJ, Hofmann IA, Bove KE. Investigation of children for mitochondriopathy confirms need for strict patient selection, improved morphological criteria, and better laboratory methods. Hum Pathol. 2006; 37:173–184. PMID: 16426917.
Article6. McFarland R, Taylor RW, Turnbull DM. Mitochondrial disease--its impact, etiology, and pathology. Curr Top Dev Biol. 2007; 77:113–155. PMID: 17222702.
Article7. Stadhouders AM, Jap PH, Winkler HP, Eppenberger HM, Wallimann T. Mitochondrial creatine kinase: a major constituent of pathological inclusions seen in mitochondrial myopathies. Proc Natl Acad Sci U S A. 1994; 91:5089–5093. PMID: 8197190.
Article8. Rustin P, Chretien D, Bourgeron T, Gérard B, Rötig A, Saudubray JM, et al. Biochemical and molecular investigations in respiratory chain deficiencies. Clin Chim Acta. 1994; 228:35–51. PMID: 7955428.
Article9. Bernier FP, Boneh A, Dennett X, Chow CW, Cleary MA, Thorburn DR. Diagnostic criteria for respiratory chain disorders in adults and children. Neurology. 2002; 59:1406–1411. PMID: 12427892.
Article10. Duman JG, Pathak NJ, Ladinsky MS, McDonald KL, Forte JG. Three-dimensional reconstruction of cytoplasmic membrane networks in parietal cells. J Cell Sci. 2002; 115:1251–1258. PMID: 11884524.
Article11. Mun JY, Lee TH, Kim JH, Yoo BH, Bahk YY, Koo HS, et al. Caenorhabditis elegans mitofilin homologs control the morphology of mitochondrial cristae and influence reproduction and physiology. J Cell Physiol. 2010; 224:748–756. PMID: 20578245.
Article12. Chow CW, Thorburn DR. Morphological correlates of mitochondrial dysfunction in children. Hum Reprod. 2000; 15(Suppl 2):68–78.
Article13. Wang X, Li H, Zheng A, Yang L, Liu J, Chen C, et al. Mitochondrial dysfunction-associated OPA1 cleavage contributes to muscle degeneration: preventative effect of hydroxytyrosol acetate. Cell Death Dis. 2014; 11. [Epub]. DOI: 10.1038/cddis.2014.473.
Article14. Yang Y, Ouyang Y, Yang L, Beal MF, McQuibban A, Vogel H, et al. Pink1 regulates mitochondrial dynamics through interaction with the fission/fusion machinery. Proc Natl Acad Sci U S A. 2008; 105:7070–7075. PMID: 18443288.
Article15. Safiulina D, Veksler V, Zharkovsky A, Kaasik A. Loss of mitochondrial membrane potential is associated with increase in mitochondrial volume: physiological role in neurones. J Cell Physiol. 2006; 206:347–353. PMID: 16110491.
Article16. Kaasik A, Safiulina D, Zharkovsky A, Veksler V. Regulation of mitochondrial matrix volume. Am J Physiol Cell Physiol. 2007; 292:C157–C163. PMID: 16870828.
Article17. Petit PX, Goubern M, Diolez P, Susin SA, Zamzami N, Kroemer G. Disruption of the outer mitochondrial membrane as a result of large amplitude swelling: the impact of irreversible permeability transition. FEBS Lett. 1998; 426:111–116. PMID: 9598989.
Article18. John GB, Shang Y, Li L, Renken C, Mannella CA, Selker JM, et al. The mitochondrial inner membrane protein mitofilin controls cristae morphology. Mol Biol Cell. 2005; 16:1543–1554. PMID: 15647377.
Article19. Zick M, Rabl R, Reichert AS. Cristae formation-linking ultrastructure and function of mitochondria. Biochim Biophys Acta. 2009; 1793:5–19. PMID: 18620004.
Article20. Cogliati S, Frezza C, Soriano ME, Varanita T, Quintana-Cabrera R, Corrado M, et al. Mitochondrial cristae shape determines respiratory chain supercomplexes assembly and respiratory efficiency. Cell. 2013; 155:160–171. PMID: 24055366.
Article21. Vogel F, Bornhövd C, Neupert W, Reichert AS. Dynamic subcompartmentalization of the mitochondrial inner membrane. J Cell Biol. 2006; 175:237–247. PMID: 17043137.
Article22. Arselin G, Vaillier J, Salin B, Schaeffer J, Giraud MF, Dautant A, et al. The modulation in subunits e and g amounts of yeast ATP synthase modifies mitochondrial cristae morphology. J Biol Chem. 2004; 279:40392–40399. PMID: 15262977.
Article23. Bustos DM, Velours J. The modification of the conserved GXXXG motif of the membrane-spanning segment of subunit g destabilizes the supramolecular species of yeast ATP synthase. J Biol Chem. 2005; 280:29004–29010. PMID: 15970598.
Article24. Paumard P, Vaillier J, Coulary B, Schaeffer J, Soubannier V, Mueller DM, et al. The ATP synthase is involved in generating mitochondrial cristae morphology. EMBO J. 2002; 21:221–230. PMID: 11823415.
Article25. von der Malsburg K, Müller JM, Bohnert M, Oeljeklaus S, Kwiatkowska P, Becker T, et al. Dual role of mitofilin in mitochondrial membrane organization and protein biogenesis. Dev Cell. 2011; 21:694–707. PMID: 21944719.26. Acehan D, Xu Y, Stokes DL, Schlame M. Comparison of lymphoblast mitochondria from normal subjects and patients with Barth syndrome using electron microscopic tomography. Lab Invest. 2007; 87:40–48. PMID: 17043667.
Article27. Mannella CA. Structure and dynamics of the mitochondrial inner membrane cristae. Biochim Biophys Acta. 2006; 1763:542–548. PMID: 16730811.
Article28. Scorrano L, Ashiya M, Buttle K, Weiler S, Oakes SA, Mannella CA, et al. A distinct pathway remodels mitochondrial cristae and mobilizes cytochrome c during apoptosis. Dev Cell. 2002; 2:55–67. PMID: 11782314.
Article29. Ding WX, Li M, Biazik JM, Morgan DG, Guo F, Ni HM, et al. Electron microscopic analysis of a spherical mitochondrial structure. J Biol Chem. 2012; 287:42373–42378. PMID: 23093403.
Article30. Mannella CA. Structural diversity of mitochondria: functional implications. Ann N Y Acad Sci. 2008; 1147:171–179. PMID: 19076440.31. Vock R, Hoppeler H, Claassen H, Wu DX, Billeter R, Weber JM, et al. Design of the oxygen and substrate basis of intracellular substrate supply to mitochondria in muscle cells. J Exp Biol. 1996; 199:1689–1697. PMID: 8708576.32. Bruno C, Bertini E, Di Rocco M, Cassandrini D, Ruffa G, De Toni T, et al. Clinical and genetic characterization of Chanarin-Dorfman syndrome. Biochem Biophys Res Commun. 2008; 369:1125–1128. PMID: 18339307.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Mitochondrial Respiratory Chain Defect Diagnosed in the Neonatal Period
- Clinical and Laboratorial Characteristics of Korean Children with Mitochondrial Respiratory Chain Defect
- Mitochondrial Respiratory Complex I Deficiency Simulating Spinal Muscular Atrophy
- Rg3 Improves Mitochondrial Function and the Expression of Key Genes Involved in Mitochondrial Biogenesis in C2C12 Myotubes
- Mitochondrial myopathies caused by prolonged use of telbivudine