J Vet Sci.
2012 Dec;13(4):345-353.
Molecular characterization of the feline T-cell receptor gamma alternate reading frame protein (TARP) ortholog
- Affiliations
-
- 1Institute of Veterinary Pathology, Justus-Liebig-Universitat Giessen, 35392 Giessen, Germany. alexander.weiss@cvua-mel.de
- 2Department of Veterinary Pathology, Freie Universitat Berlin, 14163 Berlin, Germany.
Abstract
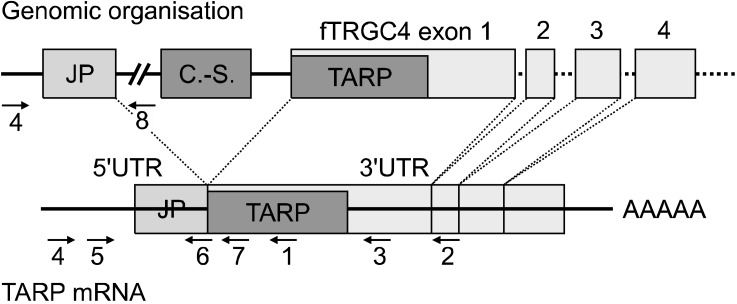
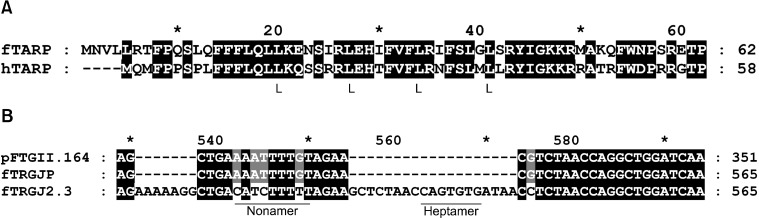
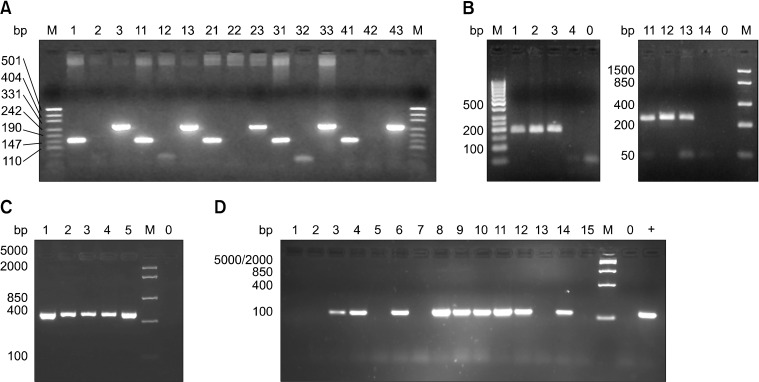
- T-cell receptor gamma alternate reading frame protein (TARP) is expressed by human prostate epithelial, prostate cancer, and mammary cancer cells, but is not found in normal mammary tissue. To date, this protein has only been described in humans. Additionally, no animal model has been established to investigate the potential merits of TARP as tumor marker or a target for adoptive tumor immunotherapy. In this study conducted to characterize feline T-cell receptor gamma sequences, constructs very similar to human TARP transcripts were obtained by RACE from the spleen and prostate gland of cats. Transcription of TARP in normal, hyperplastic, and neoplastic feline mammary tissues was evaluated by conventional RT-PCR. In felines similarly to the situation reported in humans, a C-region encoding two open reading frames is spliced to a J-region gene. In contrast to humans, the feline J-region gene was found to be a pseudogene containing a deletion within its recombination signal sequence. Our findings demonstrated that the feline TARP ortholog is transcribed in the prostate gland and mammary tumors but not normal mammary tissues as is the case with human TARP.
Keyword
MeSH Terms
Figure
Reference
-
1. Carlsson B, Tötterman TH, Essand M. Generation of cytotoxic T lymphocytes specific for the prostate and breast tissue antigen TARP. Prostate. 2004; 61:161–170. PMID: 15305339.
Article2. Cheng WS, Dzojic H, Nilsson B, Tötterman TH, Essand M. An oncolytic conditionally replicating adenovirus for hormone-dependent and hormone-independent prostate cancer. Cancer Gene Ther. 2006; 13:13–20. PMID: 16052227.
Article3. Cheng WS, Giandomenico V, Pastan I, Essand M. Characterization of the androgen-regulated prostate-specific T cell receptor γ-chain alternate reading frame protein (TARP) promoter. Endocrinology. 2003; 144:3433–3440. PMID: 12865322.
Article4. Cheng WS, Kraaij R, Nilsson B, van der Weel L, de Ridder CMA, Tötterman TH, Essand M. A novel TARP-promoter-based adenovirus against hormone-dependent and hormone-refractory prostate cancer. Mol Ther. 2004; 10:355–364. PMID: 15294182.
Article5. Cho K, Youn HY, Okuda M, Satoh H, Cevario S, O'Brien SJ, Watari T, Tsujimoto H, Hasegawa A. Cloning and mapping of cat (Felis catus) immunoglobulin and T-cell receptor genes. Immunogenetics. 1998; 47:226–233. PMID: 9435341.6. Croci S, Strippoli P, Bonsi L, Bagnara GP, Guizzunti G, Sartini R, Tonelli R, Messina C, Pierdomenico L, Lollini PL. Expression of T cell receptor alpha gene (TCRA) in human rhabdomyosarcoma and other musculo-skeletal sarcomas. Gene. 2005; 353:16–22. PMID: 15935573.
Article7. Epel M, Carmi I, Soueid-Baumgarten S, Oh SK, Bera T, Pastan I, Berzofsky J, Reiter Y. Targeting TARP, a novel breast and prostate tumor-associated antigen, with T cell receptor-like human recombinant antibodies. Eur J Immunol. 2008; 38:1706–1720. PMID: 18446790.
Article8. Essand M, Vasmatzis G, Brinkmann U, Duray P, Lee B, Pastan I. High expression of a specific T-cell receptor γ transcript in epithelial cells of the prostate. Proc Natl Acad Sci USA. 1999; 96:9287–9292. PMID: 10430935.
Article9. Fritzsche FR, Stephan C, Gerhardt J, Lein M, Hofmann I, Jung K, Dietel M, Kristiansen G. Diagnostic and prognostic value of T-cell receptor gamma alternative reading frame protein (TARP) expression in prostate cancer. Histol Histopathol. 2010; 25:733–739. PMID: 20376779.10. Jung D, Alt FW. Unraveling V(D)J recombination: insights into gene regulation. Cell. 2004; 116:299–311. PMID: 14744439.11. Jung D, Giallourakis C, Mostoslavsky R, Alt FW. Mechanism and control of V(D)J recombination at the immunoglobulin heavy chain locus. Annu Rev Immunol. 2006; 24:541–570. PMID: 16551259.
Article12. Kobayashi H, Nagato T, Oikawa K, Sato K, Kimura S, Aoki N, Omiya R, Tateno M, Celis E. Recognition of prostate and breast tumor cells by helper T lymphocytes specific for a prostate and breast tumor-associated antigen, TARP. Clin Cancer Res. 2005; 11:3869–3878. PMID: 15897588.
Article13. Krangel MS. Mechanics of T cell receptor gene rearrangement. Curr Opin Immunol. 2009; 21:133–139. PMID: 19362456.
Article14. Maeda H, Nagata S, Wolfgang CD, Bratthauer GL, Bera TK, Pastan I. The T cell receptor γ chain alternate reading frame protein (TARP), a prostate-specific protein localized in mitochondria. J Biol Chem. 2004; 279:24561–24568. PMID: 15150260.
Article15. McBlane JF, van Gent DC, Ramsden DA, Romeo C, Cuomo CA, Gellert M, Oettinger MA. Cleavage at a V(D)J recombination signal requires only RAG1 and RAG2 proteins and occurs in two steps. Cell. 1995; 83:387–395. PMID: 8521468.
Article16. Miccoli MC, Vaccarelli G, Lanave C, Cribiu EP, Ciccarese S. Comparative analyses of sheep and human TRG joining regions: evolution of J genes in Bovidae is driven by sequence conservation in their promoters for germline transcription. Gene. 2005; 355:67–78. PMID: 16039073.17. Mighell AJ, Smith NR, Robinson PA, Markham AF. Vertebrate pseudogenes. FEBS Lett. 2000; 468:109–114. PMID: 10692568.
Article19. Oh S, Terabe M, Pendleton CD, Bhattacharyya A, Bera TK, Epel M, Reiter Y, Phillips J, Linehan WM, Kasten-Sportes C, Pastan I, Berzofsky JA. Human CTLs to wild-type and enhanced epitopes of a novel prostate and breast tumor-associated protein, TARP, lyse human breast cancer cells. Cancer Res. 2004; 64:2610–2618. PMID: 15059918.
Article20. Penning LC, Vrieling HE, Brinkhof B, Riemers FM, Rothuizen J, Rutteman GR, Hazewinkel HAW. A validation of 10 feline reference genes for gene expression measurements in snap-frozen tissues. Vet Immunol Immunopathol. 2007; 120:212–222. PMID: 17904230.
Article21. Rothenfluh HS, Blanden RV, Steele EJ. Evolution of V genes: DNA sequence structure of functional germline genes and pseudogenes. Immunogenetics. 1995; 42:159–171. PMID: 7642227.
Article22. Sakano H, Hüppi K, Heinrich G, Tonegawa S. Sequences at the somatic recombination sites of immunoglobulin light-chain genes. Nature. 1979; 280:288–294. PMID: 111144.
Article23. Tonegawa S. Somatic generation of antibody diversity. Nature. 1983; 302:575–581. PMID: 6300689.
Article24. van Gent DC, McBlane JF, Ramsden DA, Sadofsky MJ, Hesse JE, Gellert M. Initiation of V(D)J recombination in a cell-free system. Cell. 1995; 81:925–934. PMID: 7781069.
Article25. Weiss ATA, Delcour NM, Meyer A, Klopfleisch R. Efficient and cost-effective extraction of genomic DNA from formalin-fixed and paraffin-embedded tissues. Vet Pathol. 2011; 48:834–838. PMID: 20817894.
Article26. Weiss ATA, Hecht W, Henrich M, Reinacher M. Characterization of C-, J- and V-region-genes of the feline T-cell receptor γ. Vet Immunol Immunopathol. 2008; 124:63–74. PMID: 18456341.
Article27. Weiss ATA, Hecht W, Reinacher M. Feline T-cell receptor γ V- and J-region sequences retrieved from the trace archive and from transcriptome analysis of cats. Vet Med Int. 2010; 2010:953272. PMID: 20634910.28. Weiss ATA, Klopfleisch R, Gruber AD. T-cell receptor γ chain variable and joining region genes of subgroup 1 are clonally rearranged in feline B- and T-cell lymphoma. J Comp Pathol. 2011; 144:123–134. PMID: 20846665.
Article29. Wolfgang CD, Essand M, Lee B, Pastan I. T-cell receptor γ chain alternate reading frame protein (TARP) expression in prostate cancer cells leads to an increased growth rate and induction of caveolins and amphiregulin. Cancer Res. 2001; 61:8122–8126. PMID: 11719440.30. Wolfgang CD, Essand M, Vincent JJ, Lee B, Pastan I. TARP: a nuclear protein expressed in prostate and breast cancer cells derived from an alternate reading frame of the T cell receptor γ chain locus. Proc Natl Acad Sci USA. 2000; 97:9437–9442. PMID: 10931945.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Full-length ORF2 sequence-based genetic and phylogenetic characterization of Korean feline caliciviruses
- Finding and Characterization of Viral Nonstructural Small Protein in Prospect Hill Virus Infected Cell
- ORF Miner: a Web-based ORF Search Tool
- Role of MAPK Signaling Pathways in Regulating the Hydrophobin Cryparin in the Chestnut Blight Fungus Cryphonectria parasitica
- A New Approach to Find Orthologous Proteins Using Sequence and Protein-Protein Interaction Similarity