Lab Anim Res.
2011 Mar;27(1):29-36. 10.5625/lar.2011.27.1.29.
Overexpression of Insulin Degrading Enzyme could Greatly Contribute to Insulin Down-regulation Induced by Short-Term Swimming Exercise
- Affiliations
-
- 1Department of Laboratory Animal Resources, National Institute of Food and Drug Evaluation, Seoul, Republic of Korea.
- 2Exercise Biochemistry Laboratory, Korea National Sport University, Seoul, Republic of Korea.
- 3Department of Biomaterials Science, College of Natural Resources & Life Science, Pusan National University, Miryang, Republic of Korea. dyhwang@pusan.ac.kr
- 4Multidisciplinary Technology Institute, Hoseo University, Asan, Republic of Korea.
- KMID: 2391855
- DOI: http://doi.org/10.5625/lar.2011.27.1.29
Abstract
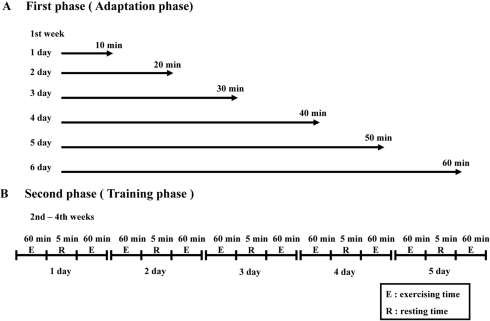
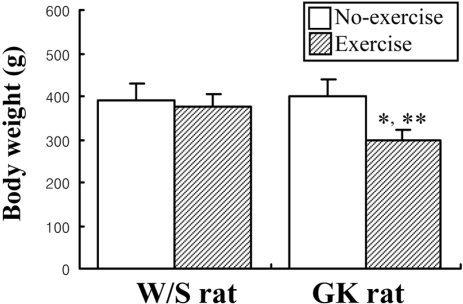
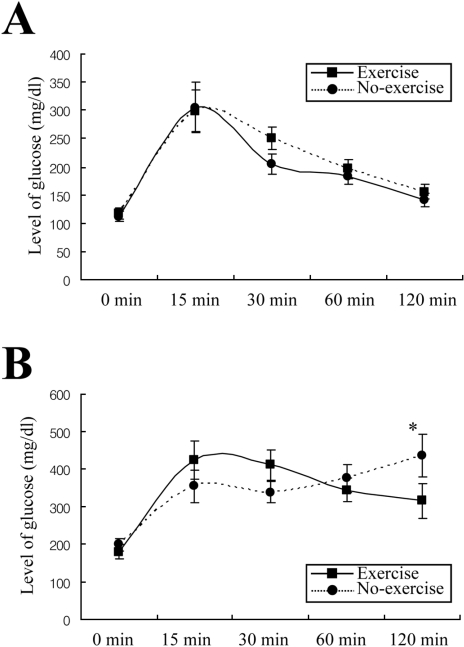
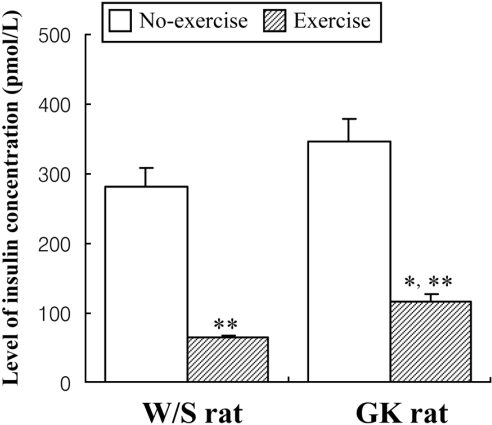
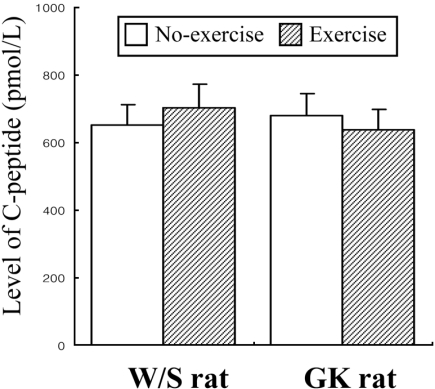
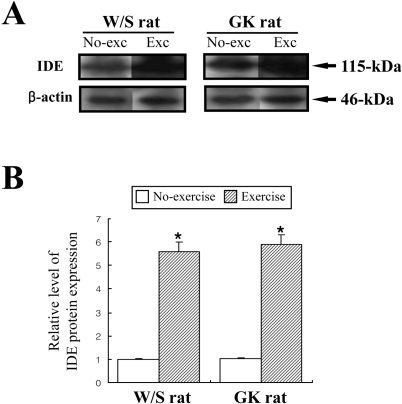
- Exercise training is highly correlated with the reduced glucose-stimulated insulin secretion (GSIS), although it enhanced insulin sensitivity, glucose uptake and glucose transporter expression to reduce severity of diabetic symptoms. This study investigated the impact of short-term swimming exercise on insulin regulation in the Goto-Kakizaki (GK) rat as a non-obese model of non-insulin-dependent diabetes mellitus. Wistar (W/S) and GK rats were trained 2 hours daily with the swimming exercise for 4 weeks, and then the changes in the metabolism of insulin and glucose were assessed. Body weight was markedly decreased in the exercised GK rats compare to their non-exercised counterpart, while W/S rats did not show any exercise-related changes. Glucose concentration was not changed by exercise, although impaired glucose tolerance was improved in GK rats 120 min after glucose injection. However, insulin concentration was decreased by swimming exercise as in the decrease of GSIS after running exercise. To identify the other cause for exercise-induced insulin down-regulation, the changes in the levels of key factors involved in insulin production (C-peptide) and clearance (insulin-degrading enzyme; IDE) were measured in W/S and GK rats. The C-peptide level was maintained while IDE expression increased markedly. Therefore, these results showed that insulin down-regulation induced by short-term swimming exercise likely attributes to enhanced insulin clearance via IDE over-expression than by altered insulin production.
Keyword
MeSH Terms
Figure
Reference
-
1. Akiyama H, Yokono K, Shii K, Ogawa W, Taniguchi H, Baba S, Kasuga M. Natural regulatory mechanisms of insulin degradation by insulin degrading enzyme. Biochem Biophys Res Commun. 1990; 170:1325–1330. PMID: 2202301.
Article2. Bennett RG, Duckworth WC, Hamel FG. Degradation of amylin by insulin-degrading enzyme. J Biol Chem. 2000; 275:36621–36625. PMID: 10973971.
Article3. Buemann B, Tremblay A. Effects of exercise training on abdominal obesity and related metabolic complications. Sports Med. 1996; 21:191–212. PMID: 8776009.
Article4. Canas X, Fernandez-Lopez JA, Ardevol A, Adan C, Esteve M, Rafecas I, Remesar X, Alemany M. Rat insulin turnover in vivo. Endocrinology. 1995; 136:3871–3876. PMID: 7649094.5. Castillo MJ, Scheen AJ, Letiexhe MR, Lefebvre PJ. How to measure insulin clearance. Diabetes Metab Rev. 1994; 10:119–150. PMID: 7956676.
Article6. Chang X, Jorgensen AM, Bardrum P, Led JJ. Solution structures of the R6 human insulin hexamer. Biochemistry. 1997; 36:9409–9422. PMID: 9235985.7. Chesneau V, Vekrellis K, Rosner MR, Selkoe DJ. Purified recombinant insulin-degrading enzyme degrades amyloid beta-protein but does not promote its oligomerization. Biochem J. 2000; 351:509–516. PMID: 11023838.8. Choi K, Kim YB. Molecular mechanism of insulin resistance in obesity and type 2 diabetes. Korean J Intern Med. 2010; 25(2):119–129. PMID: 20526383.
Article9. de Mello MA, de Souza CT, Braga LR, dos Santos JW, Ribeiro IA, Gobatto CA. Glucose tolerance and insulin action in monosodium glutamate (MSG) obese exercise-trained rats. Physiol Chem Phys Med NMR. 2001; 33:63–71. PMID: 11758736.10. de Oliveira CA, Luciano E, Marcondes MC, de Mello MA. Effects of swimming training at the intensity equivalent to aerobic/anaerobic metabolic transition in alloxan diabetic rats. J Diabetes Complicat. 2007; 21:258–264. PMID: 17616357.
Article11. de Souza CT, Nunes WM, Gobatto CA, de Mello MA. Insulin secretion in monosodium glutamate (MSG) obese rats submitted to aerobic exercise training. Physiol Chem Phys Med NMR. 2003; 35:43–53. PMID: 15139282.12. Duckworth WC, Bennett RG, Hamel FG. Insulin degradation: progress and potential. Endocr Rev. 1998; 19:608–624. PMID: 9793760.
Article13. Duckworth WC, Bennett RG, Hamel FG. The significance of intracellular insulin to insulin action. J Investig Med. 1997; 45:20–27.14. Duckworth WC. Insulin degradation: mechanisms, products, and significance. Endocr Rev. 1988; 9:319–345. PMID: 3061785.
Article15. Duckworth WC, Kitabchi AE. Insulin metabolism and degradation. Endocr Rev. 1981; 2:210–233. PMID: 7028472.
Article16. Enevoldsen LH, Stalknecht B, Fluckey JD, Galbo H. Effect of exercise training on in vivo insulin-stimulated glucose uptake in intra-abdominal adipose tissue in rats. Am J Physiol Endocrinol Metab. 2000; 278:E25–E34. PMID: 10644533.
Article17. Garcia JV, Gehm BD, Rosener MR. An evolutionarily conserved enzyme degrades transforming growth factor-alpha as well as insulin. J Cell Biol. 1989; 109:1301–1307. PMID: 2670957.
Article18. Gehm BD, Rosner MR. Regulation of insulin, epidermal growth factor, and transforming growth factor-alpha levels by growth factor-degrading enzymes. Endocrinology. 1991; 128:1603–1610. PMID: 1847863.19. Goto Y, Kakizaki M, Masaki N. Spontaneous diabetes produced by selective breeding of normal Wistar rats. Proc Jpn Acad. 1975; 51:80–85.
Article20. Hawley JA. Exercise as a therapeutic intervention for the prevention and treatment of insulin resistance. Diabetes Metab Res Rev. 2004; 20:383–393. PMID: 15343584.
Article21. Hawley JA, Lessard SJ. Exercise training-induced improvements in insulin action. Acta Physiol (Oxf). 2008; 192:127–135. PMID: 18171435.
Article22. Hwang DY, Seo SJ, Kim YK, Kim CK, Shim SB, Jee SW, Lee SH, Sin JS, Cho JY, Kang BC, Jang IS, Cho JS. Significant change in insulin production, glucose tolerance and ER stress signaling in transgenic mice coexpressing insulin-siRNA and human IDE. Int J Mol Med. 2007; 19:65–73. PMID: 17143549.
Article23. Kibenge MT, Chan CB. The effects of high-fat diet on exercise-induced changes in metabolic parameters in Zucker fa/fa rats. Metabolism. 2002; 51:708–715. PMID: 12037723.
Article24. Király MA, Bates HE, Kaniuk NA, Yue JT, Brumell JH, Matthews SG, Riddell MC, Vranic M. Swim training prevents hyperglycemia in ZDF rats: mechanisms involved in the partial maintenance of beta-cell function. Am J Physiol Endocrinol Metab. 2008; 294:E271–E283. PMID: 18029442.25. Kirschner RJ, Goldberg AL. A high molecular weight metalloendopeptidase from the cytosol of mammalian cells. J Biol Chem. 1983; 258:967–976. PMID: 6401723.26. Koranyi LI, Bourey RE, Slentz CA, Holloszy JO, Permutt MA. Coordinate reduction of rat pancreatic islet glucokinase and proinsulin mRNA by exercise training. Diabetes. 1991; 40(3):401–404. PMID: 1705526.
Article27. Marques RG, Fontaine MJ, Rogers J. C-peptide: much more than a byproduct of insulin biosynthesis. Pancreas. 2004; 29:231–238. PMID: 15367890.28. Mikines KJ, Sonne B, Tronier B, Galbo H. Effects of training and detraining on dose-response relationship between glucose and insulin secretion. Am J Physiol. 1989; 256:E588–E596. PMID: 2655469.
Article29. Moghissi ES. Insulin strategies for managing inpatient and outpatient hyperglycemia and diabetes. Mt Sinai J Med. 2008; 75:558–566. PMID: 19021195.
Article30. Morishima T, Pye S, Bradshaw C, Radziuk J. Posthepatic rate of appearance of insulin: measurement and validation in the nonsteady state. Am J Physiol. 1992; 263:E772–E779. PMID: 1415699.
Article31. Mukherjee A, Song E, Kihiko-Ehmann M, Goodman JP Jr, Pyrek JS, Estus S, Hersh LB. Insulysin hydrolyzes amyloid beta peptides to products that are neither neurotoxic nor deposit on amyloid plaques. J Neurosci. 2000; 20:8745–8749. PMID: 11102481.32. Muller D, Baumeister H, Buck F, Richter D. Atrial natriuetic peptide (ANP) is a high-affinity substrate for rat insulin-degrading enzyme. Eur J Biochem. 1991; 202:285–292. PMID: 1836994.33. Muller D, Schulze C, Baumeister H, Buck F, Richter D. Rat insulin-degrading enzyme: cleavage pattern of the natriuretic peptide hormones ANP, BNP, and CNP revealed by HPLC and mass spectrometery. Biochemistry. 1992; 31:11138–11143. PMID: 1445854.34. Ochiai M, Matsuo T. Effects of Short-Term Dietary Change from High-Carbohydrate Diet to High-Fat Diet on Storage, Utilization, and Fatty Acid Composition of Rat Muscle Triglyceride during Swimming Exercise. J Clin Biochem Nutr. 2009; 44(2):168–177. PMID: 19308271.
Article35. Oh KS, Kim EY, Yoon M, Lee CM. Swim training improves leptin receptor deficiency-induced obesity and lipid disorder by activating uncoupling proteins. Exp Mol Med. 2007; 39:385–394. PMID: 17603293.
Article36. Ostenson CG, Khan A, Abdel-Halim AM, Guenifi A, Suzuki K, Goto Y, Efendic S. Abnormal insulin secretion and glucose metabolism in pancreatic islets from the spontaneously diabetic GK rat. Diabetologia. 1993; 36:3–8. PMID: 8436249.
Article37. Ribeiro Braga L, de Mello MA, Gobatto CA. Continuous and intermittent exercise: effects of training and detraining on body fat in obese rats. Arch Latinoam Nutr. 2004; 54:58–65. PMID: 15332357.38. Safavi A, Miller BC, Cottam L, Hersh LB. Identification of gamma-endorphin-generating enzyme as insulin-degrading enzyme. Biochemistry. 1996; 35:14318–14325. PMID: 8916918.39. Seta KA, Roth RA. Overexpression of insulin degrading enzyme: cellular localization and effects on insulin signaling. Biochem Biophys Res Commun. 1997; 231:167–171. PMID: 9070242.
Article40. Sonksen P, Sonksen J. Insulin: understanding its action in health and disease. Br J Anaesth. 2000; 85:69–79. PMID: 10927996.
Article41. Suzuki KI, Goto Y, Toyota T. Shafrir E, editor. Spntaneously diabetic GK (Goto-Kakizaki) rats. Lessons from Animal Diabetes. 1992. 1st ed. London: Smith-Gordon;p. 107–116.42. Ueda H, Urano Y, Sakurai T, Kizaki T, Hitomi Y, Ohno H, Izawa T. Enhanced expression of neuronal nitric oxide synthase in islets of exercise-trained rats. Biochem Biophys Res Commun. 2003; 312(3):794–800. PMID: 14680835.
Article43. Valera Mora ME, Scarfone A, Calvani M, Greco AV, Mingrone G. Insulin clearance in obesity. J Am Coll Nutr. 2003; 22:487–493. PMID: 14684753.
Article44. Vekrellis K, Ye Z, Qiu WQ, Walsh D, Hartley D, Chesneau V, Rosner MR, Selkoe DJ. Neurons regulate extracellular levels of amyloid beta-protein via proteolysis by insulin-degrading enzyme. J Neurosci. 2000; 20:1657–1665. PMID: 10684867.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Exercise on Neuropathy in Streptozotocin-Induced Diabetic Rats
- AMPK and Exercise: Glucose Uptake and Insulin Sensitivity
- Change of platelet aggregation after short-term exercise in non- insulin dependent diabetics
- Role of insulin-like growth factor binding protein-3 in glucose and lipid metabolism
- Effect of Exercise Training on Insulin Sensitivity and Intracellular Glucose Metabolism in Skeletal Muscle of High Fat-fed Rats