Ann Rehabil Med.
2017 Jun;41(3):402-412. 10.5535/arm.2017.41.3.402.
Effects of Exercise on Neuropathy in Streptozotocin-Induced Diabetic Rats
- Affiliations
-
- 1Department of Physical and Rehabilitation Medicine, Inha University, Incheon, Korea. rmkmo@inha.ac.kr
- KMID: 2389455
- DOI: http://doi.org/10.5535/arm.2017.41.3.402
Abstract
OBJECTIVE
To evaluate the effects of early regular exercise and to assess the electrophysiological and histopathological findings of the rat tail nerve in relation to the timing of exercise training for swimming exercise in rats with diabetic neuropathy.
METHODS
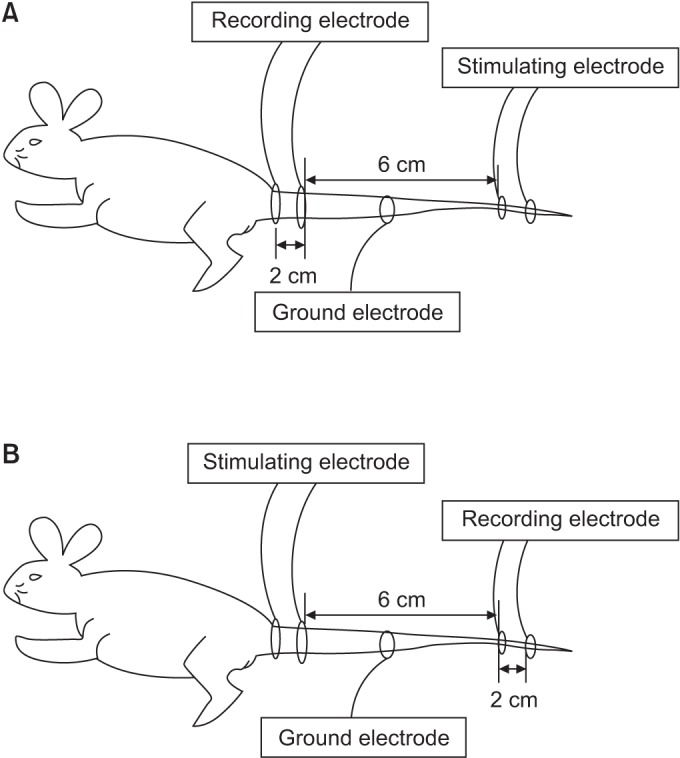
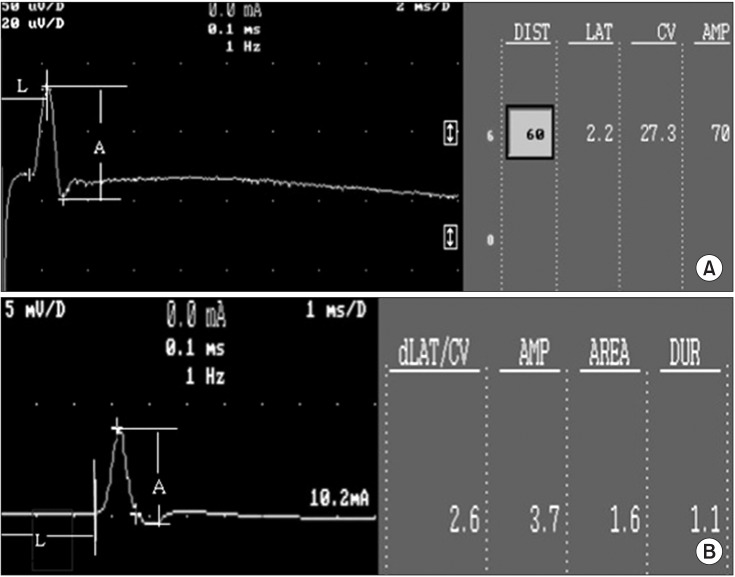
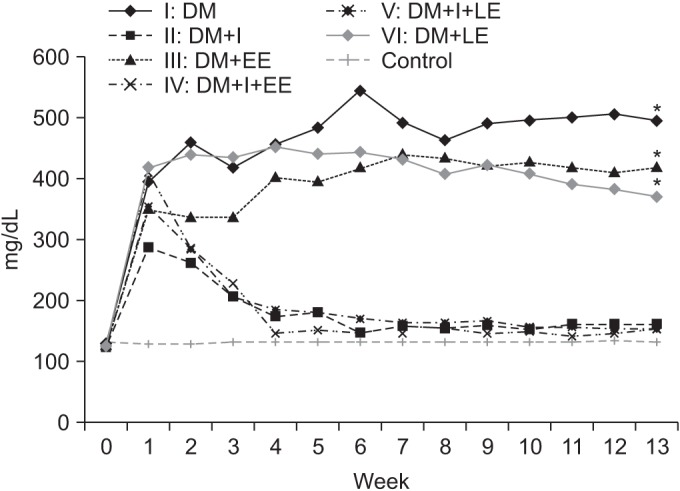
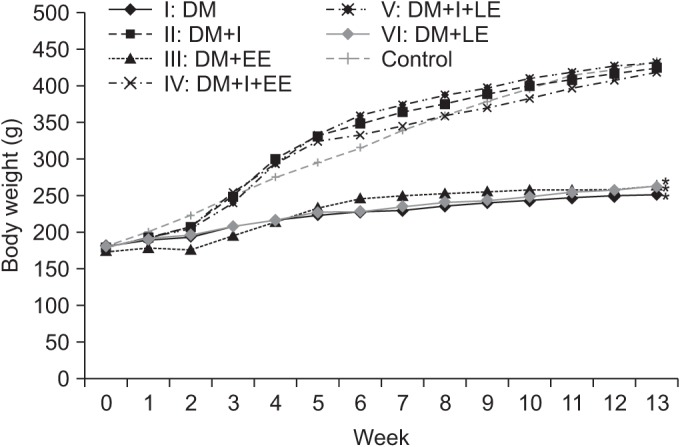
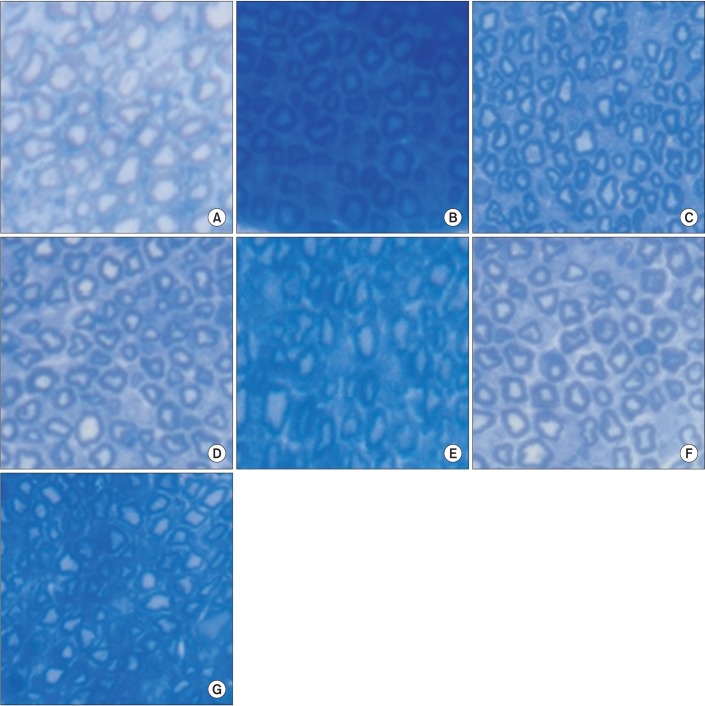
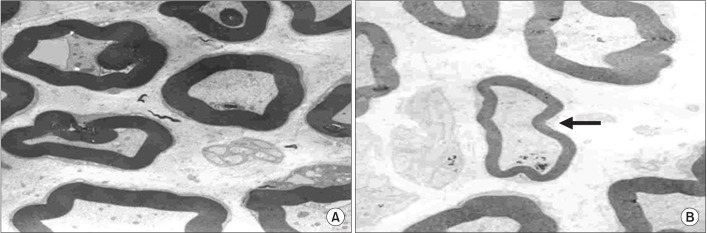
We used 70 Sprague-Dawley male rats, and the experimental group comprised 60 rats, and the control group comprised 10 rats. Diabetes was induced by intraperitoneal injection of streptozotocin. Blood glucose concentrations were measured in tail vein blood samples. The experimental group was divided into 6 subgroups according to insulin treatment and swimming exercise: group 1, diabetic control; group 2, insulin treated; group 3, insulin untreated with early swimming exercise; group 4, insulin treated and early swimming exercise; group 5, insulin treated and late swimming exercise; and group 6, insulin untreated with late swimming exercise. Sensory and motor nerve conduction studies were performed weekly up to the 13th week using rat tail nerves. The effect on structural diabetic neuropathy was assessed by morphometry and ultrastructural examination of the rat tail nerve fiber at the 14th week.
RESULTS
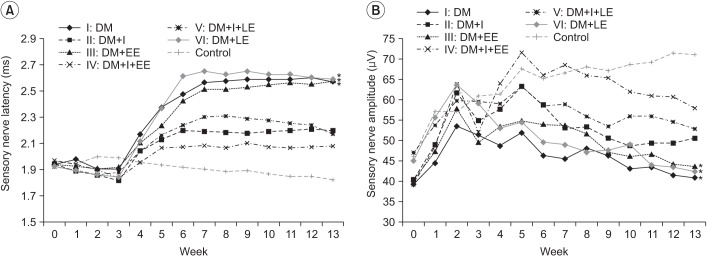
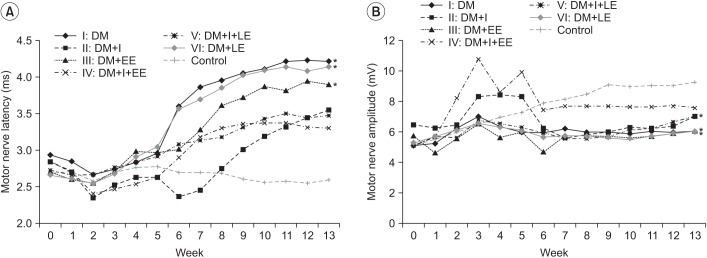
An exercise effect was observed in the insulin treated groups, but it was not observed in the insulin untreated groups. The sensory nerve conduction study in the rat tail revealed significantly prolonged latency and decreased amplitude in groups 1 and 6, and a further delay was observed in group 5 when compared to group 4. Decreased thickness of myelin was found in groups 1 and 6 through morphometry.
CONCLUSION
Early regular exercise programs in addition to conventional insulin treatment may retard the progression of diabetic peripheral neuropathy.
MeSH Terms
Figure
Reference
-
1. Pyun SB, Kwon HK, Uhm CS. Effect of exercise on reinnervating soleus muscle after sciatic nerve injury in rats. J Korean Acad Rehabil Med. 1999; 23:1063–1075.2. Dumitru D. Electrodiagnostic medicine. 1st ed. Philadelphia: Mosby;1995. p. 821–824.3. Dyck PJ, Thomas PK. Diabetic neuropathy. 2nd ed. Philadelphia: W.B. Saunders;1999. p. 222–236. p. 330–340.4. Singleton JR, Smith AG, Marcus RL. Exercise as therapy for diabetic and prediabetic neuropathy. Curr Diab Rep. 2015; 15:120. PMID: 26538074.
Article5. Malysz T, Ilha J, Nascimento PS, De Angelis K, Schaan BD, Achaval M. Beneficial effects of treadmill training in experimental diabetic nerve regeneration. Clinics (Sao Paulo). 2010; 65:1329–1337. PMID: 21340223.
Article6. van Meeteren NL, Brakkee JH, Helders PJ, Wiegant VM, Gispen WH. Functional recovery from sciatic nerve crush lesion in the rat correlates with individual differences in responses to chronic intermittent stress. J Neurosci Res. 1997; 48:524–532. PMID: 9210522.
Article7. Kwon HK, Lee HJ, Yim SK, Lee SR. Electrophysiologic assessement of axonopathy and demyelination in diabetic neuropathy according to the severity. J Korean Acad Rehabil Med. 2002; 26:50–54.8. Kim MO, Kim SJ, Choi HC, Roh GH, Kim SY. Histochemical findings of soleus in relation to the severity of injury and duration of exercise in sciatic nerve injured rats. J Korean Acad Rehabil Med. 2003; 27:727–734.9. Abu-Shakra SR, Cornblath DR, Avila OL, Chaudhry V, Freimer M, Glass JD, et al. Conduction block in diabetic neuropathy. Muscle Nerve. 1991; 14:858–862. PMID: 1922181.
Article10. Said G. Diabetic neuropathy: a review. Nat Clin Pract Neurol. 2007; 3:331–340. PMID: 17549059.11. Park DW, Nam KS, Kim SC, Park SI, Choi E, Lee YG. Significance of amplitude and area ratio of compound muscle action potential in diagnosis of diabetic neuropathy. J Korean Acad Rehabil Med. 2001; 25:615–620.12. Park BK, Kim SJ. Response of peripheral nerve to transient ischemia in streptozotocin-induced diabetic rats. J Korean Acad Rehabil Med. 1993; 17:392–405.13. Kim CH, Choi HC, Roh GH. The preventive effect of nimodipine on the cisplatin induced neuropathy. J Korean Acad Rehabil Med. 2003; 27:90–95.14. Gulsen I, Demiroglu M, Aycan A, Ucler R, Alaca I, Orhon ZN, et al. Effects of low-intensity treadmill exercise on sciatic nerve in experimental diabetic neuropathy. Anal Quant Cytopathol Histpathol. 2016; 38:95–102. PMID: 27386630.15. Kim WS, Lee SU. Harmful effect of land-based endurance exercise in rats with diabetic nerve. Med Sci Sports Exerc. 2010; 42:1625–1631. PMID: 20142779.
Article16. Kim MO, Yoon JS, Kwak JR, Choi HC, Roh GH, Kim SJ. Effect of therapeutic exercise according to degree of injury in sciatic nerve damaged rat. J Korean Acad Rehabil Med. 2001; 25:466–473.17. Dyck PJ, Davies JL, Wilson DM, Service FJ, Melton LJ 3rd, O'Brien PC. Risk factors for severity of diabetic polyneuropathy: intensive longitudinal assessment of the Rochester Diabetic Neuropathy Study cohort. Diabetes Care. 1999; 22:1479–1486. PMID: 10480512.
Article18. Eugene B, Abthony SF, Dennis LK, Stephen LH, Dan LL. Harrison's principles of internal medicine. 15th ed. McGraw-Hill;2001. p. 2109–2137.19. Hotta N, Koh N, Sakakibara F, Nakamura J, Hamara Y, Hara T, et al. Effects of propionyl-L-carnitine and insulin on the electroretinogram, nerve conduction and nerve blood flow in rats with streptozotocin-induced diabetes. Pflugers Arch. 1996; 431:564–570. PMID: 8596700.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Protective Effect of Melatonin on Neuropathy in Streptozotocin-Induced Diabetic Rats
- Proliferation of Cultured Vascular Smooth Muscle Cells(VSMCs) Obtained from Aortas of Insulin Dependent Diabetic Rats
- The Effects of Nerve Growth Factor on Satellite Cell of the Dorsal Rott Ganglia in Streptozotocin-induced Diabetic Rats
- Alteration of Nerve Growth Factor in the Penis of Rats with Streptozotocin-induced Diabetes
- The Morphological Studies on the Effects of Nerve Growth Factor on the Schwann Cell in the Diabetic Neuropathy in the Rats