Lab Anim Res.
2017 Sep;33(3):216-222. 10.5625/lar.2017.33.3.216.
Early changes in retinal structure and BMP2 expression in the retina and crystalline lens of streptozotocin-induced diabetic pigs
- Affiliations
-
- 1Department of Ophthalmology and Inha Vision Science Laboratory, Inha University School of Medicine, Incheon, Korea. drmys@inha.ac.kr, nrkim@inha.ac.kr
- 2Laboratory of Developmental Genetics, Department of Biomedical Sciences, Inha University School of Medicine, Incheon, Korea.
- KMID: 2391094
- DOI: http://doi.org/10.5625/lar.2017.33.3.216
Abstract
- PURPOSE
This study aims to evaluate early changes in retinal structure and BMP2 expression in the retina and crystalline lens by comparing streptozotocin-induced diabetic pigs and normal control group pigs.
METHODS
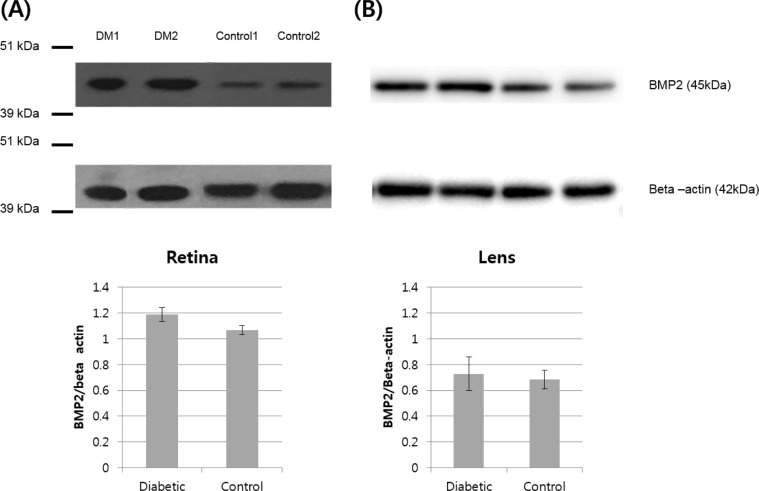
Five eye samples from five diabetic Micro-pigs (Medikinetics, Pyeongtaek, Korea) and five eye samples from five control pigs bred in a specific pathogen-free area were used. Diabetes was developed through intravenous injection of nicotinamide and streptozotocin, and the average fasting glucose level was maintained at 250 mg/dL or higher for 16 weeks. To evaluate BMP2 expression in the retina and crystalline lens, Western blotting was performed.
RESULTS
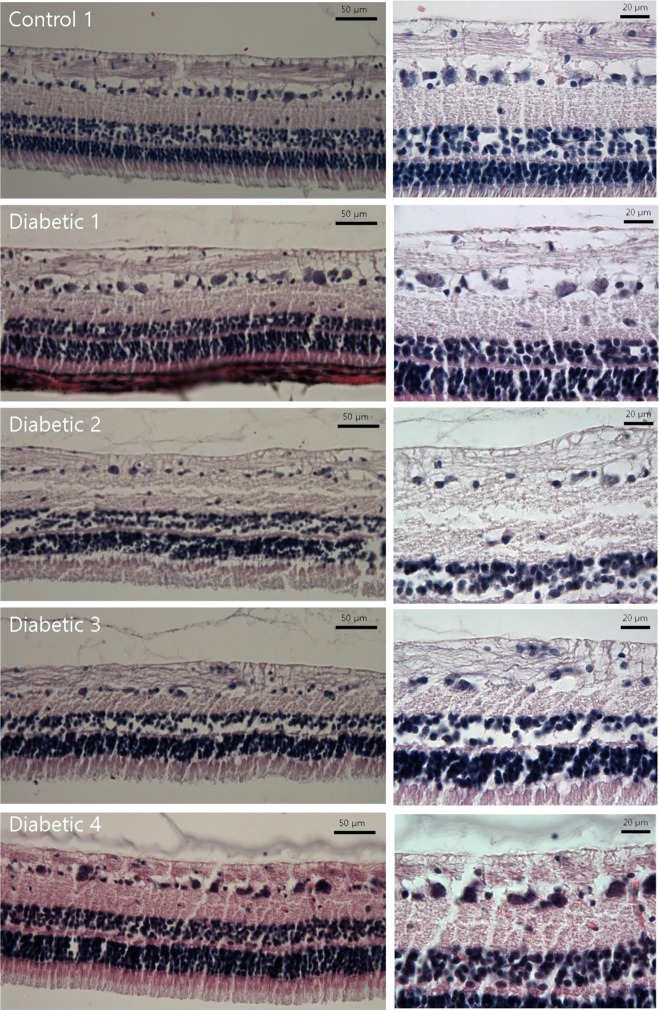
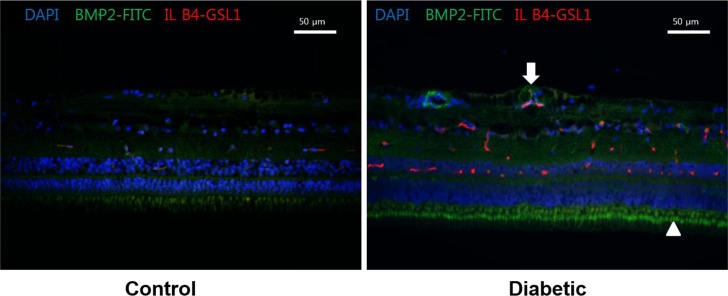
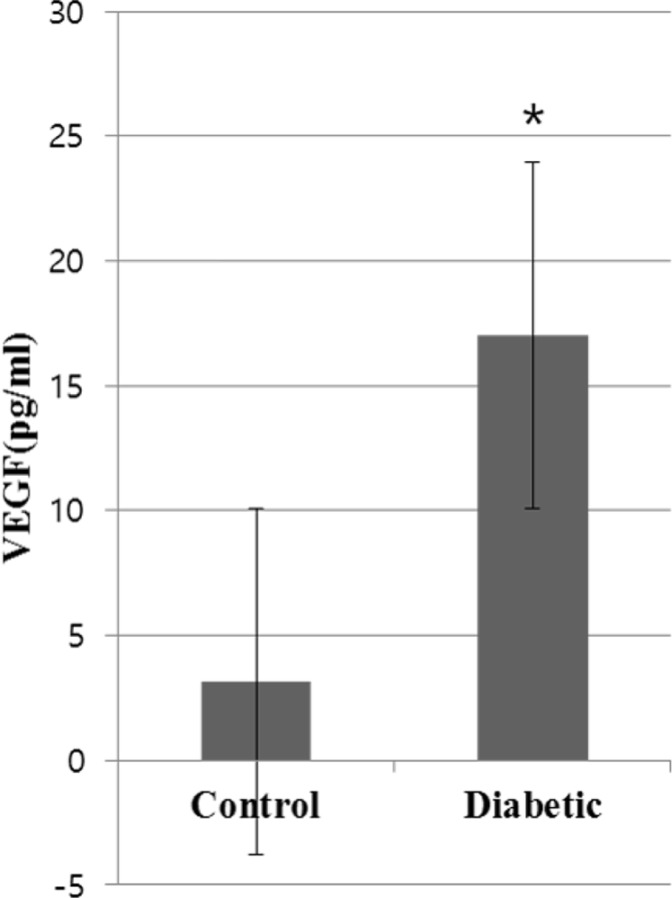
In Hematoxylin and Eosin staining, most diabetic pigs showed structural abnormalities in the inner plexiform layer. The number of nuclei in the ganglion cell layer within the range of 10â´µm² was 3.78±0.60 for diabetic pigs and 5.57±1.07 for control group pigs, showing a statistically significant difference. In immunohistochemical staining, diabetic retinas showed an overall increase in BMP2 expression. In Western blotting, the average BMP2/actin level of diabetic retinas was 1.19±0.05, showing a significant increase compared to the 1.06±0.03 of the control group retinas (P=0.016). The BMP2/actin level of diabetic crystalline lenses was similar to the control group crystalline lenses (P=0.730).
CONCLUSIONS
Compared to control group pigs, the number of nuclei in the inner nuclear layer of retinas from streptozotocin-induced diabetic pigs decreased, while an increase in BMP2 expression was observed in the retina of diabetic pigs.
Keyword
MeSH Terms
Figure
Reference
-
1. Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund J, Haffner S, Hamman RF, Ikram MK, Kayama T, Klein BE, Klein R, Krishnaiah S, Mayurasakorn K, O'Hare JP, Orchard TJ, Porta M, Rema M, Roy MS, Sharma T, Shaw J, Taylor H, Tielsch JM, Varma R, Wang JJ, Wang N, West S, Xu L, Yasuda M, Zhang X, Mitchell P, Wong TY. Meta-Analysis for Eye Disease (META-EYE) Study Group. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012; 35(3):556–564. PMID: 22301125.
Article2. van Dijk HW, Verbraak FD, Kok PH, Garvin MK, Sonka M, Lee K, Devries JH, Michels RP, van Velthoven ME, Schlingemann RO, Abràmoff MD. Decreased retinal ganglion cell layer thickness in patients with type 1 diabetes. Invest Ophthalmol Vis Sci. 2010; 51(7):3660–3665. PMID: 20130282.
Article3. Sohn EH, van Dijk HW, Jiao C, Kok PH, Jeong W, Demirkaya N, Garmager A, Wit F, Kucukevcilioglu M, van Velthoven ME, DeVries JH, Mullins RF, Kuehn MH, Schlingemann RO, Sonka M, Verbraak FD, Abràmoff MD. Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc Natl Acad Sci U S A. 2016; 113(19):E2655–E2664. PMID: 27114552.
Article4. Guo J, Wu G. The signaling and functions of heterodimeric bone morphogenetic proteins. Cytokine Growth Factor Rev. 2012; 23(1-2):61–67. PMID: 22421241.
Article5. Akeel S, El-Awady A, Hussein K, El-Refaey M, Elsalanty M, Sharawy M, Al-Shabrawey M. Recombinant bone morphogenetic protein-2 induces up-regulation of vascular endothelial growth factor and interleukin 6 in human pre-osteoblasts: role of reactive oxygen species. Arch Oral Biol. 2012; 57(2):445–452. PMID: 22041018.
Article6. Hussein KA, Choksi K, Akeel S, Ahmad S, Megyerdi S, El-Sherbiny M, Nawaz M, Abu El-Asrar A, Al-Shabrawey M. Bone morphogenetic protein 2: a potential new player in the pathogenesis of diabetic retinopathy. Exp Eye Res. 2014; 125:79–88. PMID: 24910902.
Article7. Lee MS, Song KD, Yang HJ, Solis CD, Kim SH, Lee WK. Development of a type II diabetic mellitus animal model using Micropig®. Lab Anim Res. 2012; 28(3):205–208. PMID: 23091521.
Article8. van Dijk HW, Kok PH, Garvin M, Sonka M, Devries JH, Michels RP, van Velthoven ME, Schlingemann RO, Verbraak FD, Abràmoff MD. Selective loss of inner retinal layer thickness in type 1 diabetic patients with minimal diabetic retinopathy. Invest Ophthalmol Vis Sci. 2009; 50(7):3404–3409. PMID: 19151397.
Article9. Park HS, Park SJ, Park SH, Chun MH, Oh SJ. Shifting of parvalbumin expression in the rat retina in experimentally induced diabetes. Acta Neuropathol. 2008; 115(2):241–248. PMID: 17989985.
Article10. Kern TS, Barber AJ. Retinal ganglion cells in diabetes. J Physiol. 2008; 586(18):4401–4408. PMID: 18565995.
Article11. Zhang M, Zhou SH, Zhao S, Li XP, Liu LP, Shen XQ. Pioglitazone can downregulate bone morphogenetic protein-2 expression induced by high glucose in human umbilical vein endothelial cells. Pharmacology. 2008; 81(4):312–316. PMID: 18311072.
Article12. Belecky-Adams TL, Adler R, Beebe DC. Bone morphogenetic protein signaling and the initiation of lens fiber cell differentiation. Development. 2002; 129(16):3795–3802. PMID: 12135918.
Article13. Boswell BA, Overbeek PA, Musil LS. Essential role of BMPs in FGF-induced secondary lens fiber differentiation. Dev Biol. 2008; 324(2):202–212. PMID: 18848538.
Article14. Perry RE, Swamy MS, Abraham EC. Progressive changes in lens crystallin glycation and high-molecular-weight aggregate formation leading to cataract development in streptozotocin-diabetic rats. Exp Eye Res. 1987; 44(2):269–282. PMID: 3582512.
Article15. Chung SS, Ho EC, Lam KS, Chung SK. Contribution of polyol pathway to diabetes-induced oxidative stress. J Am Soc Nephrol. 2003; 14:S233–S236. PMID: 12874437.
Article16. Hussein KA, Zakhary IE, Elawady AR, Emam HA, Sharawy M, Baban B, Akeel S, Al-Shabrawey M, Elsalanty ME. Difference in soft tissue response between immediate and delayed delivery suggests a new mechanism for recombinant human bone morphogenetic protein 2 action in large segmental bone defects. Tissue Eng Part A. 2012; 18(5-6):665–675. PMID: 21981405.
Article17. Mathura JR Jr, Jafari N, Chang JT, Hackett SF, Wahlin KJ, Della NG, Okamoto N, Zack DJ, Campochiaro PA. Bone morphogenetic proteins-2 and -4: negative growth regulators in adult retinal pigmented epithelium. Invest Ophthalmol Vis Sci. 2000; 41(2):592–600. PMID: 10670493.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Vascular Endothelial Growth Factor (VEGF) and Advanced Glycation End Products (AGEs) Overexpression in the Retina and Serum and Lens Opacities of Streptozotocin-induced Diabetic Rats
- NADPH Diaphorase Staining Retinal Cells in Streptozotocin-induced Diabetic Rat Retina
- An Ultrastructural Study on the Early Morphologic Changes of the Retina in Streptozotocin-induced Diabetic Rats
- A Case of Crystalline Retinopathy
- Alterations of Tyrosine Hydroxylase and Choline Acetyltransferase in the Retina of the Diabetic Rat