Pediatr Gastroenterol Hepatol Nutr.
2017 Sep;20(3):167-177. 10.5223/pghn.2017.20.3.167.
Safety of a New Synbiotic Starter Formula
- Affiliations
-
- 1Department of Pediatrics, Universitair Ziekenhuis Brussel, Vrije Universiteit Brussel, Brussels, Belgium. Yvan.Vandenplas@uzbrussel.be
- 2Department of Hygiene, Epidemiology and Medical Statistics, Faculty of Medicine, National and Kapodistrian University of Athens, Athens, Greece.
- 33rd Pediatric Department, Hippocration Hospital, Thessaloniki, Greece.
- KMID: 2390548
- DOI: http://doi.org/10.5223/pghn.2017.20.3.167
Abstract
- PURPOSE
Breastfeeding is the best way to feed all infants, but not all infants can be (exclusively) breastfed. Cow's milk based infant formula is the second choice infant feeding.
METHODS
The safety of a new synbiotic infant formula, supplemented with Bifidobacterium lactis and fructo-oligosaccharides, with lactose and a whey/casein 60/40 protein ratio was tested in 280 infants during 3 months.
RESULTS
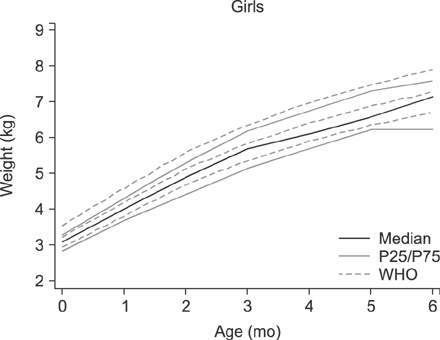
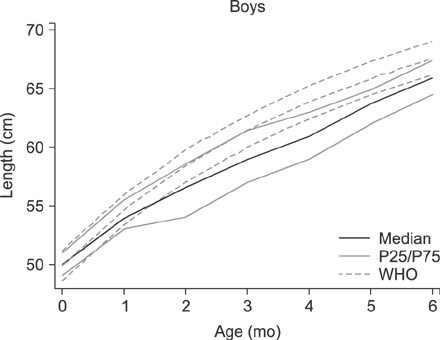
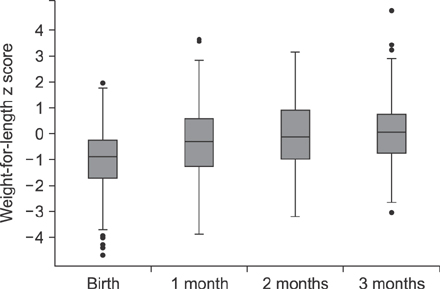
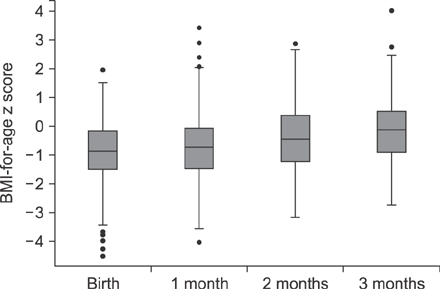
The median age of the infants at inclusion was 0.89 months. Weight evolution was in accordance with the World Health Organization growth charts for exclusive breastfed infants. The evolution of all anthropometric parameters (weight-for-length z score and body mass index-for-age z score) was within the normal range. The incidence of functional constipation (3.2%), daily regurgitation (10.9%), infantile crying and colic (10.5%) were all significantly lower than the reported median prevalence for a similar age according to literature (median value of 7.8% for functional constipation, 26.7% for regurgitation, 17.7% for infantile colic).
CONCLUSION
The new synbiotic infant starter formula was safe, resulted in normal growth and was well tolerated. Functional gastro-intestinal manifestations (functional constipation, regurgitation and colic) were significantly lower than reported in literature. Synbiotics (Bifidobacterium lactis and fructo-oligosaccharides) in cow's milk based infant formula bring the second choice infant feeding, formula, closer to the golden standard, exclusive breastfeeding.
Keyword
MeSH Terms
Figure
Reference
-
1. Field CJ. The immunological components of human milk and their effect on immune development in infants. J Nutr. 2005; 135:1–4.
Article2. Garcia C, Duan RD, Brévaut-Malaty V, Gire C, Millet V, Simeoni U, et al. Bioactive compounds in human milk and intestinal health and maturity in preterm newborn: an overview. Cell Mol Biol (Noisy-le-grand). 2013; 59:108–131.3. Jost T, Lacroix C, Braegger C, Chassard C. Impact of human milk bacteria and oligosaccharides on neonatal gut microbiota establishment and gut health. Nutr Rev. 2015; 73:426–437.
Article4. Wopereis H, Oozeer R, Knipping K, Belzer C, Knol J. The first thousand days-intestinal microbiology of early life: establishing a symbiosis. Pediatr Allergy Immunol. 2014; 25:428–438.
Article5. Kapiki A, Costalos C, Oikonomidou C, Triantafyllidou A, Loukatou E, Pertrohilou V. The effect of a fructo-oligosaccharide supplemented formula on gut flora of preterm infants. Early Hum Dev. 2007; 83:335–339.
Article6. Bäckhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe. 2015; 17:690–703.
Article7. Reisinger KW, de Vaan L, Kramer BW, Wolfs TG, van Heurn LW, Derikx JP. Breast-feeding improves gut maturation compared with formula feeding in preterm babies. J Pediatr Gastroenterol Nutr. 2014; 59:720–724.
Article8. Vandenplas Y, Zakharova I, Dmitrieva Y. Oligosaccharides in infant formula: more evidence to validate the role of prebiotics. Br J Nutr. 2015; 113:1339–1344.
Article9. Szajewska H, Chmielewska A. Growth of infants fed formula supplemented with Bifidobacterium lactis Bb12 or Lactobacillus GG: a systematic review of randomized controlled trials. BMC Pediatr. 2013; 13:185.
Article10. Steenhout PG, Rochat F, Hager C. The effect of Bifidobacterium lactis on the growth of infants: a pooled analysis of randomized controlled studies. Ann Nutr Metab. 2009; 55:334–340.
Article11. Braegger C, Chmielewska A, Decsi T, Kolacek S, Mihatsch W, Moreno L, et al. Supplementation of infant formula with probiotics and/or prebiotics: a systematic review and comment by the ESPGHAN committee on nutrition. J Pediatr Gastroenterol Nutr. 2011; 52:238–250.
Article12. Huet F, Abrahamse-Berkeveld M, Tims S, Simeoni U, Beley G, Savagner C, et al. Partly fermented infant formulae with specific oligosaccharides support adequate infant growth and are well-tolerated. J Pediatr Gastroenterol Nutr. 2016; 63:e43–e53.
Article13. Weaver LT, Ewing G, Taylor LC. The bowel habit of milk-fed infants. J Pediatr Gastroenterol Nutr. 1988; 7:568–571.
Article14. Bocquet A, Lachambre E, Kempf C, Beck L. Effect of infant and follow-on formulas containing B lactis and galacto-and fructo-oligosaccharides on infection in healthy term infants. J Pediatr Gastroenterol Nutr. 2013; 57:180–187.
Article15. Vandenplas Y, De Greef E, Veereman G. Prebiotics in infant formula. Gut Microbes. 2014; 5:681–687.
Article16. Holscher HD, Czerkies LA, Cekola P, Litov R, Benbow M, Santema S, et al. Bifidobacterium lactis Bb12 enhances intestinal antibody response in formula-fed infants: a randomized, double-blind, controlled trial. JPEN J Parenter Enteral Nutr. 2012; 36:1 Suppl. 106S–117S.17. Indrio F, Di Mauro A, Riezzo G, Civardi E, Intini C, Corvaglia L, et al. Prophylactic use of a probiotic in the prevention of colic, regurgitation, and functional constipation: a randomized clinical trial. JAMA Pediatr. 2014; 168:228–233.
Article18. Vandenplas Y, Abkari A, Bellaiche M, Benninga M, Chouraqui JP, Çokura F, et al. Prevalence and health outcomes of functional gastrointestinal symptoms in infants from birth to 12 months of age. J Pediatr Gastroenterol Nutr. 2015; 61:531–537.
Article19. Savino F, Maccario S, Castagno E, Cresi F, Cavallo F, Dalmasso P, et al. Advances in the management of digestive problems during the first months of life. Acta Paediatr Suppl. 2005; 94:120–124.
Article20. Ben XM, Li J, Feng ZT, Shi SY, Lu YD, Chen R, et al. Low level of galacto-oligosaccharide in infant formula stimulates growth of intestinal Bifidobacteria and Lactobacilli. World J Gastroenterol. 2008; 14:6564–6568.
Article21. Veereman-Wauters G, Staelens S, Van de, Plaskie K, Wesling F, Roger LC, et al. Physiological and bifidogenic effects of prebiotic supplements in infant formulae. J Pediatr Gastroenterol Nutr. 2011; 52:763–771.
Article22. Scholtens PA, Alliet P, Raes M, Alles MS, Kroes H, Boehm G, et al. Fecal secretory immunoglobulin A is increased in healthy infants who receive a formula with short-chain galacto-oligosaccharides and long-chain fructo-oligosaccharides. J Nutr. 2008; 138:1141–1147.
Article23. Iacono G, Merolla R, D'Amico D, Bonci E, Cavataio F, Di Prima L, et al. Gastrointestinal symptoms in infancy: a population-based prospective study. Dig Liver Dis. 2005; 37:432–438.
Article24. Giovannini M, Verduci E, Gregori D, Ballali S, Soldi S, Ghisleni D, et al. Prebiotic effect of an infant formula supplemented with galacto-oligosaccharides: randomized multicenter trial. J Am Coll Nutr. 2014; 33:385–393.
Article25. Savino F, Palumeri E, Castagno E, Cresi F, Dalmasso P, Cavallo F, et al. Reduction of crying episodes owing to infantile colic: a randomized controlled study on the efficacy of a new infant formula. Eur J Clin Nutr. 2006; 60:1304–1310.
Article26. Gerasimov SV, Vasjuta VV, Myhovych OO, Bondarchuk LI. Probiotic supplement reduces atopic dermatitis in preschool children: a randomized, double-blind, placebo-controlled, clinical trial. Am J Clin Dermatol. 2010; 11:351–361.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Small-bowel bezoars in an infant following synbiotic ingestion: a novel case report
- Can a Synbiotic Supplementation Contribute to Decreasing Anti-Tissue Transglutaminase Levels in Children with Potential Celiac Disease?
- A Synbiotic Infant Formula with High Magnesium Content Improves Constipation and Quality of Life
- Growth and Tolerance Assessment of a Lutein-fortified Infant Formula
- Combination of synbiotic and sitagliptin in nonalcoholic fatty liver disease: Is it better than sitagliptin alone?